

Surgical patients undergoing general anesthesia have been extensively studied; fatal pulmonary embolism (PE) rates range from 0.1% to 0.8% for all patients[1, 2] and may be as high as 7% for patients undergoing surgery for fractured hips.[3] Many different forms of therapy have been evaluated in this group. Studies of pneumatic compression in cardiac surgery and neurosurgical patients have shown a distinct improvement in the incidence of deep venous thrombosis (DVT) without the added risk of bleeding.[4, 5] However, the effect is less impressive in higher-risk patients, and compliance can be difficult.
Timing and duration of prophylactic agents has also been determined to have a significant effect the development of DVT. Early prophylaxis in surgical patients with low-molecular-weight heparin (LMWH) has been associated with significant reductions in postoperative venous thrombosis. A study by Hull et al found that initiation of therapy within 8 hours of surgery had the greatest effect.[6] The ninth edition of the clinical practice guidelines for prevention of venous thromboembolism (VTE) from the American College of Chest Physicians (ACCP) recommended that LMWH be given to patients undergoing major orthopedic procedures at least 12 hours preoperatively or postoperatively.[7]
For more information, see Deep Venous Thrombosis and Pulmonary Embolism.
NextMechanical methods have been shown to be a useful adjunct to anticoagulation therapy in reducing the incidence of DVT. Modalities include passive devices, such as knee- or thigh-high graduated compression (elastic) stockings (GCS)[8] ; active (external pneumatic compress or intermittent pneumatic compression [IPC]) devices[9] ; or venous foot pumps (VFP).[10]
A 2012 systematic review of randomized, controlled trials found that knee- and thigh-high GCS do not significantly differ in their effectiveness in reducing the incidence of DVT in hospitalized patients. Ease of use, patient compliance, and cost influence which type of stocking is used in clinical practice.[11]
In a study of the efficacy of IPC in multiple postoperative patient groups versus no use of prophylaxis, Urbankova et al reported that the incidence of DVT was reduced by 60%.[12] However, the use of mechanical means of prophylaxis alone is not effective in moderate or high-risk cases.
IPC devices are designed to decrease venous stasis, improve blood flow velocity, and increase the level of circulating fibrinolysins. IPC devices have the advantage of requiring no monitoring, with no increase in bleeding. Generally, they are well tolerated. There are a wide variety of these devices, and they can be applied to the foot, calf, or thigh. A study comparing asymmetrical with circumferential intermittent compression devices following total knee replacement (TKR) seemed to support the asymmetrical device.[13]
Patient compliance is an issue with IPC devices, and the efficacy is dependent on the time of use. Evidence from clinical trials has also shown that although the rate of distal thrombi is reduced significantly, proximal thrombi are not. This finding may lead to a false sense of security because although the total number of deep venous thrombi may be similar to the numbers observed with pharmacologic prophylaxis, the proportion of the relatively more dangerous proximal clots is increased (see Table 1 below).
Table 1. Results of Intermittent Pneumatic Compression Versus Warfarin (Open Table in a new window)
Thrombi Warfarin (n = 72) IPC (n = 67) Iliac and femoral 5 14 Calf, popliteal, plantar 10 2 Total 15 16Although all three types of mechanical compression reduce the incidence of DVT to less than that found when prophylaxis is absent, these modalities are generally less effective at producing such reductions than are pharmacologic methods. Shorter lengths of hospital stays make the use of mechanical methods alone ineffective in preventing DVT in the critical weeks after joint replacement. No mechanical prophylaxis method has been shown to reduce the risk of PE or death. The use of IPC devices is therefore recommended primarily as an adjunct to anticoagulant-based prophylaxis or in patients who are at high risk of bleeding.
Many pharmacologic agents are currently available to prevent thrombosis. Agents that retard or inhibit the process belong under the general heading of anticoagulants. Agents that prevent the growth or formation of thrombi are properly termed antithrombotics and include anticoagulants and antiplatelet drugs, whereas thrombolytic drugs lyse existing thrombi. For the importance of prevention, see Hull and Pineo's 1998 study.[14, 15, 16]
Platelet-active drugs such as aspirin or cyclooxygenase (COX)-1 inhibitors have been used to prevent thrombosis.[17] Aspirin is effective as a platelet inhibitor at very low dosages (50-100 mg/day). This dosage is significantly less than that necessary to produce an anti-inflammatory effect. However, a meta-analysis of the effect of aspirin following total hip replacement (THR) completed in 1994 had equivocal results.[18, 19]
A large study performed in Europe, the Pulmonary Embolism Prevention (PEP) study, found that the overall DVT rate was decreased 30% with low-dose aspirin compared with placebo, and the overall pulmonary thrombosis rate was decreased by 40%. This trial included 13,356 patients with hip fractures and 4088 patients with THR.[19] Aspirin at 160 mg/day was compared with placebo and evaluated at day 35. Approximately 40% of the patients also were given low-density heparin or LMWH.[19]
In a concomitant study of 4088 patients with THRs, a 25% reduction of DVT was observed in comparison with the placebo control, but no decrease was noted in the rate of pulmonary embolism.[19] This trial did not show a clear benefit to using aspirin as the primary method of venous prophylaxis in patients undergoing either total hip or total knee surgery.
The Seventh ACCP Conference did not recommend the use of aspirin alone as a prophylactic agent for any patient group, because aspirin is less effective than other options. However, reports by Lotke and Lonner[20] and by Berend and Lombardi[21] suggested that the use of aspirin combined with optimally used IPC devices may be effective in some circumstances in preventing fatal pulmonary embolism.
Coumarins are a class of oral anticoagulant drugs, which act as antagonists to vitamin K. The mechanism of action is to interfere with the interaction between vitamin K and coagulation factors II, VII, IX, and X. Vitamin K acts as a cofactor at these levels. Coumarins produce their anticoagulant effect by inhibiting the carboxylation necessary for biologic activity.
Warfarin is a mixture of two isomers, the R and S forms, in roughly equal proportions. This agent is absorbed rapidly from the gastrointestinal (GI) tract and bound to plasma proteins. Although it has high bioavailability, warfarin requires 36-72 hours to reach a stable loading dose. The dose response in patients taking warfarin is variable, and it is influenced by various genetic and environmental factors. In addition, numerous drug interactions and disease states may affect its pharmacokinetics. Warfarin, therefore, requires continuous laboratory monitoring.
The effectiveness of warfarin anticoagulation is measured by determining the prothrombin time (PT) or protime against a standard control. The use of the international normalized ratio (INR) has supplanted the PT for hospital use. INR uses a standardized PT, which allows for comparisons between hospitals and laboratories.
For DVT prophylaxis, the optimal INR is between 2 and 3, with a target of 2.5. When used for DVT prophylaxis after THR, warfarin reduces total DVT by 60% and proximal DVT by 70%. Disadvantages of warfarin use include its long onset of action, the necessity to monitor INR values frequently to obtain a stable dosage, the long half-life that may require vitamin K reversal in incidents of hemorrhage, frequent drug and dietary interaction, and variable patient response. Hemorrhagic complications are reported in up to 3-5% of patients on warfarin prophylaxis.
If adjusted-dose warfarin is to be used, it is started the night before surgery and continued postoperatively during the discharge period. INR target levels are not usually reached until postoperative day 3.
Standard unfractionated heparin (UFH) is recognized as an acceptable anticoagulant modality and has been used for this purpose in various forms since its discovery by McLean in 1916. UFH acts in conjunction with a circulating plasma cofactor, antithrombin (AT) III and, in its presence, catalyzes the inactivation of factors IIa, Xa, IXa, and XIIa.
By inactivating thrombin, heparin not only prevents fibrin formation but also inhibits thrombin-induced activation of factor V and factor VIII. Of these, factors IIa and Xa are most sensitive. Therefore, heparin has anticoagulant and antithrombotic properties.
Heparin is a heterogeneous mixture of molecules that contain a range of molecular weights of 3-30 kd, with an average of approximately 15 kd. Only one third of the heparin molecules have an active binding site for ATIII, and this fraction is responsible for most of the anticoagulant activity.
Heparin is effective when given by intravenous (IV) or subcutaneous (SC) administration but is inactivated in the GI tract. This agent has a rapid onset of action, its half-life is brief in comparison to warfarin, and it binds to platelets, endothelial cells, and macrophages in vivo. Therapeutic levels of heparin are measured by the activated partial thromboplastin time (aPTT). Because of the rapid clearance of heparin from the bloodstream, therapeutic levels (aPTT of 1.2-1.5 × control) are more likely achieved with continuous IV infusion.
Postoperative DVT prophylaxis with UFH is usually achieved by administering a bolus of 5000 U every 8 hours. This low-dose heparin regimen results in a 60-70% reduction of DVT and PE in low-risk or moderate-risk patients. However, this method is not as effective in patients who are at high risk for development of DVT or PE. In these patients, adjusted-dose heparin with aPTT monitoring is preferred to maintain the desired anticoagulant level. Studies have demonstrated a high hemorrhagic complication rate of 8-15% when this method is used for postoperative DVT prophylaxis.
Heparin overdosage is reversible with protamine sulfate, which itself is an anticoagulant. Each milligram of protamine sulfate can neutralize approximately 100 U of heparin activity. This agent must be administered very slowly by IV infusion over a 10-minute period in doses not to exceed 50 mg. Because heparin is rapidly cleared from the circulation, the amount of protamine required decreases rapidly as the time from initial heparin administration increases. The final dosage required is titrated according to coagulation studies.
Disadvantages of UFH therapy include the following:
In addition, a small percentage of patients (2-4%) are susceptible to the development of heparin-induced thrombocytopenia (HIT), which is an antibody-mediated adverse reaction that can cause venous and arterial thrombosis. HIT is heralded by an otherwise unexpected fall in platelet count of greater than 50% from previous levels. HIT can result in disseminated intravascular coagulation (DIC) and gangrene in severe cases. Treatment with danaparoid sodium or recombinant hirudin, such as lepirudin, may be effective in life-threatening cases.
A comparison study by McGarry et al of outcomes of thromboprophylaxis between an LMWH (enoxaparin) and UFH revealed a 74% lower incidence of VTE in the LMWH group.[22] No significant difference in side effects, deaths in the hospital, or economics was noted.
LMWHs are manufactured when standard heparin is treated by various enzymatic or chemical methods to select those lower-molecular-weight moieties that contain the active ATIII binding site. The average molecular weight of fractionated heparin is 4.5 kd rather than the usual 15 kd. The molecular-weight threshold under which anti-factor Xa activity is maximized is 5.4 kd.
The polysaccharide side chain of the heparin molecule is decreased from 18 U to approximately 13 U. As the length of the side chain is decreased, the ability of the molecule to prolong the aPTT is lost, but the ability to complex with ATIII is retained. LMWHs do not require monitoring of either aPTT or INR (see the image below).
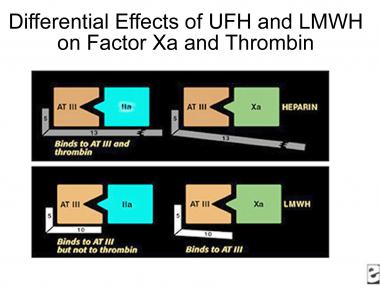 Comparison of binding sites for standard heparin and low-molecular-weight heparin.
Comparison of binding sites for standard heparin and low-molecular-weight heparin.
The pharmacologic effect of this transformation is to make the LMWH more bioavailable (~90%, compared with 29% for UFH) and to lengthen its half-life to 4 hours from 1 hour for UFH. LMWH also increases the activity ratio of anti-Xa to anti-IIa, resulting in increased antithrombotic activity.
In experimental models and animal studies, LMWH produces less microvascular bleeding than UFH, but this finding has not been duplicated in human trials. Compared with placebo, LMWHs produced a 70-80% risk reduction for DVT in numerous studies without an increase in major bleeding in high-risk orthopedic patients. Meta-analyses comparing various other methods of DVT prophylaxis, including low-dose UFH, adjusted-dose heparin, and warfarin, have demonstrated improvement in DVT prophylaxis without increase in hemorrhagic complications.[23, 24, 25]
Several LMWH medications are commercially available, including the following:
Fondaparinux sodium, a synthetic pentasaccharide, selectively binds to ATIII and potentiates neutralization of factor Xa, inhibiting thrombin formation and thrombus development.[27] Fondaparinux acts rapidly but has a long half-life (18 hours). A dose of 2.5 mg SC daily can be started 6-8 hours postoperatively. Renal clearance requires a minimal kidney function of creatinine clearance (CrCl) of greater than 30 mL/min or a weight of over 110 lb. In a controlled study by Bauer et al, fondaparinux was more effective than enoxaparin in preventing DVT after TKR, but episodes of major bleeding were more frequent.[28] For a comparison between fondaparinux and enoxaparin, see Turpie et al.[29]
Keeney and colleagues reported on the use of early mobilization with a combination of IPC and adjusted-dose short-duration warfarin in a group of patients undergoing 700 primary and revision total hip arthroplasties.[30] The investigators recorded a low incidence of clinical DVT (as measured by ultrasonography) on postoperative day 3 or 4 and of clinical DVT and PE within 90 days postoperatively. Further clinical investigations with larger numbers of patients are necessary to determine the optimal levels and duration of anticoagulation with the appropriate risk/benefit ratio.
Large phase III clinical trials have described the use of the factor Xa inhibitor rivaroxaban for prevention of thromboembolism following total knee or total hip arthroplasty. Rivaroxaban is administered once daily and has shown significant superiority in preventing DVT, nonfatal PE, or death compared with SC enoxaparin following arthroplastic surgery. It was the first orally active direct inhibitor of coagulation factor Xa approved by the US Food and Drug Administration (FDA) and is indicated for prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery.[31, 32, 33, 34, 35, 36]
Rivaroxaban was compared with enoxaparin for acute DVT. Efficacy showed noninferiority to enoxaparin for short-term use. When used as continued treatment, rivaroxaban had superior efficacy (P <.001) compared with placebo. A study comparing rivaroxaban to warfarin for long-term efficacy is warranted.[37]
Rivaroxaban was found to be equivalent to enoxaparin/vitamin K antagonist in the treatment of established DVT, also without an increase in bleeding complications.[38]
Another factor Xa inhibitor, apixaban, was approved by the FDA in March 2014 for postoperative prophylaxis of DVT/PE following hip or knee arthroplasty. Apixaban compared favorably with enoxaparin in the prevention of DVT after hip or knee replacement without increased bleeding.[39, 40]
For more information, see Deep Venous Thrombosis and Pulmonary Embolism.
In November 2015, the FDA approved dabigatran for prophylaxis of DVT and PE after hip replacement surgery.[41] Approval was based on the RE-NOVATE and RE-NOVATE II trials.[42, 43] Dabigatran was found to have similar effectiveness and safety in preventing VTE following hip arthroplasty as compared with enoxaparin.
In separate studies, Rosendaal, Kearon, and Bulger et al analyzed the relative contribution of individual risk factors to the development of DVT.[44, 45, 46] When more than one risk factor is present, the risk is cumulative; however, no good model suggests how the individual risk factors interact.[47] Nonetheless, several attempts have been made to quantify the risk factors associated with VTE.[48] The use of a checklist to stratify patients and assign them to categories of relative propensity for DVT development is helpful in deciding on an appropriate treatment regimen (see below).
A list can be constructed by using the ACCP risk categories.[49] . These figures include a list of the pertinent factors, which are arbitrarily assigned a risk level between 1 and 5. An individual aged 61-75 years is assigned 2 units; a person older than 75 years is assigned a score of 3, as is an individual with a previous history of thrombosis, inherited thrombophilia, antiphospholipid antibodies, or lupusanticoagulant.The total score is then added.
Risk factors are grouped according to severity and are added to produce an overall risk factor score, which corresponds to a low through a very high potential for DVT development.
In risk factor assessment, 1 point is assigned to each of the following:
Each of the following risk factors is assigned 2 points:
The following risk factors are assigned 3 points each:
Finally, 5 points are assigned to each of the following risk factors:
These factors include those that diminish venous flow or return, increase viscosity, or alter mobility. Age is one of the most easily definable factors.[50] The risk of DVT increases in exponential fashion with increasing age (see the image below).
 Time course of deep venous thrombosis risk.
Time course of deep venous thrombosis risk.
By using the risk criteria listed above, orthopedic patients can be categorized into four risk groups, ranging from low to very high (see Table 2 below). Appropriate methods of prophylaxis may be applied to each level.
Table 2. Deep Venous Thrombosis Risk Factor Scores (Open Table in a new window)
Risk Factor Score 0-1 2 3-4 5+ DVT Incidence 2% 10-20% 20-40% 40-80% Risk level Low Moderate High Very highLow-risk patients have a score of 1 or less. These are individuals who are younger than 40 years who are undergoing a minor surgical procedure and have no additional risk factors. The risk of calf DVT in this group is estimated to be 2-5% without prophylaxis, and the risk of clinical pulmonary thrombosis is 0.2% No specific prophylaxis is required in this group other than early and aggressive mobilization.
Moderate-risk patients have a score of 2 or less. They are individuals in the above group who have additional risk factors or are aged 40-60 years who are undergoing nonmajor surgery and have no additional risk factors. Other risk factors are surgery requiring a tourniquet (eg, arthroscopy), lower-extremity fractures, cast immobilization, or spinal surgery.
Major surgery in patients younger than 40 years poses a moderate risk of DVT, which is estimated at 10-20%. The risk of clinical PE in this group is 1-2%. Successful prevention strategies in this group consist of low-dose UFH (LDUH; q12hr), LMWH (<3400 U q24hr), and GCS or IPC.
High-risk patients have a score of 3 or 4 and include persons older than 60 years, as well as patients aged 40-60 years who have additional risk factors, such as previous VTE, malignancy, or hypercoagulability. The risk of calf DVT is estimated at 20-40% in this group, with clinical pulmonary embolism occurring in 2-4%. Successful prevention strategies in this group consist of LDUH (q8hr) and LMWH (>3400 U q24hr), with or without IPC.
The highest-risk patients have a score of 5 or greater; they are older than 40 years who have additional risk factors, who are undergoing hip or knee replacement surgery, or who have had hip fracture, open lower-leg fracture, multiple trauma, or spinal cord injury. Hip fracture patients have the highest risk of dying from a fatal PE. Additional risk factors may include a history of VTE, malignancy, or hypercoagulable state. These factors carry an estimated risk of calf DVT of 40-80% without prophylaxis, with clinical PE occurring in 4-10% and fatal PE in 0.2-5%.
Successful prevention strategies include LMWH (>3400 U q24hr), fondaparinux, and coumarins (INR 2-3). Dose-adjusted LDUH or LMWH may be used with or without IPC/GCS.
To see complete information on Deep Venous Thrombosis Risk Stratification, please go to the main article by clicking here.
In 2012, the American College of Chest Physicians (ACCP) issued recommendations for VTE prevention in orthopedic surgery patients, based on the ninth edition of its evidence-based clinical practice guidelines for antithrombotic therapy and prevention of thrombosis.[7]
Recommendations for patients undergoing major orthopedic surgery (total hip arthroplasty [THA], total knee arthroplasty [TKA], or hip fracture surgery [HFS]) included the following[7] :
In patients undergoing knee arthroscopy who do not have a prior history of VTE, no thromboprophylaxis was recommended.[7]
For patients who have no additional risk factors, antithrombotic prophylaxis following elective spine surgery is not recommended. Patients at high risk for developing postoperative VTE may be treated with LDUH, LMWH, or perioperative IPC. Multiple risk factors may require the combined use of mechanical and pharmacologic measures.
Epstein reported a 2.8% incidence of DVT and a 0.7% incidence of PE in 139 patients following multilevel lumbar spine surgery treated with IPC and early mobilization.[51] For prevention of thromboembolism in spinal cord injuries, see the recommendations of the Consortium for Spinal Cord Medicine.[52]
Anticoagulant prophylaxis should be used with caution in patients receiving spinal or indwelling catheter epidural anesthesia. Although the risk of spinal hematoma is very small (0.0025% with spinal anesthesia and 0.03% with epidural anesthesia), care should be taken to delay the initiation of thromboprophylaxis for at least 2 hours after catheter removal. Patients with known bleeding disorders should not receive preoperative prophylaxis if they are to receive spinal anesthesia. In cases of traumatic spinal tap with bloody spinal fluid, postoperative administration of thromboprophylaxis should be done with caution.
In Europe, it is common practice to begin anticoagulant prophylaxis 10-12 hours before surgery. In North America, the practice is to begin treatment 12-24 hours following surgery. The ninth edition of the ACCP guidelines suggested that for most major elective orthopedic surgical procedures, the first dose of LMWH may be administered either before or after surgery,[7] though meta-analyses suggest little advantage for preoperative initiation.
A study by Bergqvist and Hull seemed to suggest that starting a half-dose of anticoagulation 6 hours after surgery may deliver more effective prophylaxis without a significant increase in bleeding risk.[53] Patients with a high risk of bleeding should have the first postoperative dose delayed 12-24 hours after surgery. In a meta-analysis of 33 trials, Leonardi et al reported an approximately 3% rate of bleeding complications from DVT prophylaxis in which the bleeding was severe enough to require a change of care.[54]
For additional information, see Deep Venous Thrombosis, and Pulmonary Embolism.
An ideal anticoagulant should be easy to administer (preferably oral), should be effective and safe with a minimum of possible complications or adverse effects, have a rapid onset, have a therapeutic half-life, and require minimal or no monitoring. The action of the anticoagulant should be predictable with few drug or dietary interactions, and it should be reversible. The drug should also be inexpensive. Unfortunately, these criteria are often difficult to achieve. Several anticoagulant agents exist today, and each of them incorporates some of these characteristics, but no single agent combines all these attributes.
Current research in anticoagulants involves investigations into drugs that act on various phases of the coagulation cascade. For convenience, the authors can arbitrarily divide the process into three phases: the initiation phase, the propagation phase, and the thrombin activity phase.
Drugs under investigation that act in the initiation phase include tissue factor pathway inhibitors (TFPIs) and nematode anticoagulant peptide (NAPc2). These drugs act through inhibition of the factor VIIa/tissue factor complex.
A number of newer synthetic direct or indirect inhibitors of thrombin or factor Xa are being tested. These have similarities to the currently approved fondaparinux. Phase II dose-finding trials in which the metapentasaccharide idraparinux was administered subcutaneously (SC) once each week to prevent the development of secondary venous thromboembolism (VTE) have been completed. A second class of orally active direct factor Xa inhibitors, which includes razaxaban, is also undergoing clinical phase II trials.[55]
Ximelagatran, a direct thrombin inhibitor consisting of an oral prodrug of melagatran, is rapidly absorbed through the GI tract, where it is converted to its active form, melagatran. It does not require monitoring, as it has a rapid onset of action, a predictable dose-response, and a therapeutic half-life. Also, like the other direct thrombin inhibitors, it does not affect the aPTT or PT.
The results reported by Francis et al in the New England Journal of Medicine showed that ximelagatran and warfarin did not differ significantly with respect to the incidence of major or minor bleeding.[56] The report also determined that ximelagatran was significantly more effective in preventing DVT after TKR than was warfarin.
In the United States, four studies showed that postoperatively initiated ximelagatran (24 mg twice daily) had efficacy similar to that of enoxaparin or warfarin in the prevention of VTE in patients undergoing hip or knee replacement. Overall, the incidence of bleeding events and transfusion rates were not markedly different from those of comparator anticoagulants. Some patients experienced transient elevations of liver enzyme levels, which returned to normal after cessation of treatment.[57]
The FDA Cardiovascular and Renal Drug Advisory Committee (CRAC) reviewed the ximelagatran clinical program to propose the following three indications[58] :
The committee reviewed data from 30,698 subjects and included five phase III studies, which led the CRAC to use the finding of ximelagatran hepatic toxicity as a key feature for an unfavorable benefit/risk ratio of ximelagatran for the three proposed indications. A report by Colwell and Mouret, however, indicated that melagatran/ximelagatran has been approved in the European Union for the prevention of VTE in patients undergoing elective hip or knee replacement surgery.[59] Boudes reported on the challenges and risk analysis to be learned from the ximelagatran FDA CRAC findings.[58]
Drugs that act on the third stage of the coagulation cascade, the thrombin activity phase, include the direct thrombin inhibitors. These drugs specifically inactivate thrombin and are independent of ATII). Included in this group are the hirudins and their derivatives made by recombinant DNA techniques.
Originally, hirudin was isolated from leech salivary gland tissue. The new drugs include bivalirudin and lepirudin. A randomized, multicenter, double-blind study of hirudin versus heparin in patients with THRs demonstrated that DVT and proximal DVT rates were decreased substantially in the hirudin group.
Another class of drugs acting at the third level of the coagulation cascade includes the noncovalent inhibitor argatroban, which is a carboxylic acid derivative that has been approved for use in the treatment of HIT.
In a randomized, controlled study of 90 patients undergoing total knee arthroplasty, Izumi et al found that intraoperative transcutaneous electrical nerve stimulation (TENS) had a significant effect with regard to prevention of DVT prophylaxis, preventing both venous stasis and blood hypercoagulability.[60]
For additional information, see Deep Venous Thrombosis, and Pulmonary Embolism.
Major surgical and high-risk orthopedic procedures place patients at risk for DVT and VTE, including PE. Complications of DVT include postphlebitic syndrome or death from PE. Therefore, prophylaxis with anticoagulant medications, as well as the adjunctive use of mechanical devices, is essential. The most effective treatment protocol for a patient must be determined on a case-by-case basis and account for the risk-benefit ratio in each situation. A risk stratification protocol, such as that developed by the ACCP, is recommended to determine the appropriate level and method of treatment.
Preventing VTE is always a tradeoff between the potential life-saving benefit of prophylaxis and the risk of hemorrhage. Therefore, even with adequate anticoagulant prophylaxis, DVT can and does develop. A study by Schiff et al revealed a 14% incidence of VTE following major orthopedic procedures, particularly TKR, in which standard prophylactic measures had been applied.[61] Continued vigilance and a high index of suspicion on the part of the medical staff is called for in this group of patients.
Please see complete information on Deep Venous Thrombosis, by visiting the main article.
Please see complete information on Pulmonary Embolism by visiting the main article.
Copyright © www.orthopaedics.win Bone Health All Rights Reserved