

Spinal cord disease results from multiple diverse pathologic processes. Trauma is the most common cause of spinal cord injury. Depending on its pathogenesis, spinal cord disease can manifest with variable impairment of motor, sensory, or autonomic function. This review focuses on spinal cord anatomy. Basic clinical descriptions of common patterns of spinal cord involvement are related to essential aspects of spinal cord anatomy.
The spinal cord is located inside the vertebral canal, which is formed by the foramina of 7 cervical, 12 thoracic, 5 lumbar, and 5 sacral vertebrae, which together form the spine. The spinal cord extends from the foramen magnum down to the level of the first and second lumbar vertebrae (at birth, down to second and third lumbar vertebrae). See the image below.
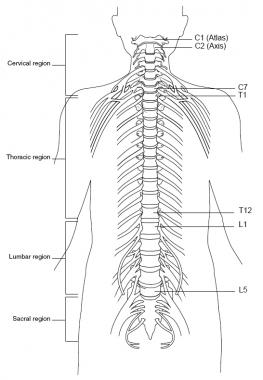 Spine, anterior view.
Spine, anterior view.
The spinal cord is composed of the following 31 segments:
The spinal nerves consist of the sensory nerve roots, which enter the spinal cord at each level, and the motor roots, which emerge from the cord at each level. The spinal nerves are named and numbered according to the site of their emergence from the vertebral canal. C1-7 nerves emerge above their respective vertebrae. C8 emerges between the seventh cervical and first thoracic vertebrae. The remaining nerves emerge below their respective vertebrae. See the image below.
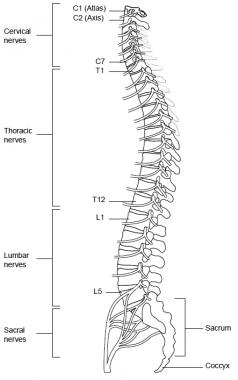 Spine, lateral view.
Spine, lateral view.
The dorsal rami of C1-4 are located in the suboccipital region. C1 participates in the innervation of neck muscles, including the semispinalis capitis muscle. C2 carries sensation from the back of the head and scalp, along with motor innervation to several muscles in the neck. C3-C5 contribute to the formation of the phrenic nerve and innervate the diaphragm. C5-T1 provide motor control for the upper extremities and related muscles.
The thoracic cord has 12 segments and provides motor control to the thoracoabdominal musculature. The lumbar and sacral portions of the cord have 5 segments each. L2-S2 provide motor control to lower extremities and related muscles.
The conus medullaris is the cone-shaped termination of the caudal cord. The pia mater continues caudally as the filum terminale through the dural sac and attaches to the coccyx. The coccyx has only 1 spinal segment. The cauda equina (Latin for horse tail) is the collection of lumbar and sacral spinal nerve roots that travel caudally prior to exiting at their respective intervertebral foramina. The cord ends at vertebral levels L1-L2.
NextThe cell body (ie, soma) is in the anterior horn within the cord parenchyma. Clinically relevant reflex center levels are as follows (spinal reflex center levels are presented in parentheses and take into account anatomic variations in innervation):
The cell bodies of the sensory nerves are located in the dorsal root ganglia. Each dorsal root contains the input from all the structures within the distribution of its corresponding body segment (ie, somite). Dermatomal maps portray sensory distributions for each level. These maps differ somewhat according to the methods used in their construction.
Charts based on injection of local anesthetics into single dorsal root ganglia show bands of hypalgesia to be continuous longitudinally from the periphery to the spine. Maps derived from other methods, such as observation of herpes zoster lesion distributions or surgical root section, show discontinuous patterns. In addition, innervation from one dermatomal segment to another overlaps considerably, more so for touch than for pain. As the dermatomes travel from the back to the chest and abdomen, they tend to dip inferiorly.
Clinically important dermatomes are as follows:
Several macroscopic grooves are discernible on the surface of the spinal cord. Most prominent is the anterior median fissure, which is occupied by the anterior spinal artery. The posterior median sulcus is less prominent. The anterior and posterior nerve rootlets emerge at the anterolateral and posterolateral sulci.
The external part of the spinal cord consists of white matter, while the internal part is composed of gray matter. The white matter includes the 3 funiculi: posterior, lateral, and anterior. Each contains ascending and descending tracts. A tract is usually named by a composite of its origin and destination; for example, the corticospinal tract originates at the cerebral cortex and ends at the spinal cord.
The gray matter can be divided into 10 laminae/layers or into 4 parts: anterior or ventral horn (ie, motor neurons; laminae VIII, IX, and part of VII), posterior or dorsal horn (ie, sensory part; laminae I-VI), intermediate zones (ie, associate neurons; lamina VII), and lateral horns (ie, part of the intermediate zone, present in the thoracic and lumbar segments, where sympathetic neurons are located).
The spinal gray matter neurons are also arranged into columns or nuclei. The substantia gelatinosa and proper sensory nucleus extend throughout the whole spinal cord and receive pain impulses. Other nuclei, such as the nucleus of Clarke, are present only in certain segments.
Corticospinal tract
This largest and most important descending tract exists in the cerebral cortex and is composed of over 1 million fibers (700,000 are myelinated, 90% with a diameter of 1-4 µm). Fibers arise from neurons in the deeper lamina V in the precentral gyrus (Brodmann area 4), premotor area (area 6), postcentral gyrus (areas 1, 2, 3, and 3b), and adjacent parietal cortex (area 5). Only a small part of the corticospinal tract (CST) originates from the large pyramidal cortical neurons, the 34,000-40,000 Betz cells in each hemisphere (Brodmann area 4). However, most of the large fibers (10-20 µm) originate there. See the image.
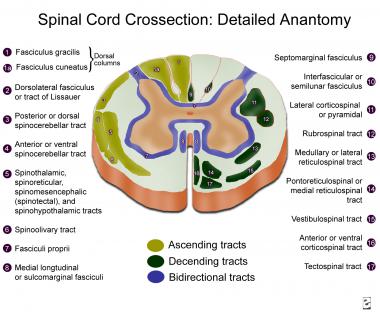 Spinal cord cross-section, detailed anatomy.
Spinal cord cross-section, detailed anatomy.
CST fibers arise from cells arranged in strips of different sizes. Their axons converge in the corona radiata, enter the internal capsule, and form the crus cerebri in the midbrain. In the medulla, they form the pyramids; at the junction of the medulla and spinal cord, the CST incompletely decussates (75-90% of the fibers), forming the large, lateral CST; the small, anterior CST (uncrossed); and the small, uncrossed, anterolateral CST.
Fibers of the lateral CST run through the lateral funiculus, medial to the posterior spinothalamic tract and lateral to the fasciculus proprius. Axons from the lateral CST synapse with alpha motor neurons and interneurons (more commonly) from laminae IV, V, VI, and VII. The lateral CST decreases in size at the more caudal spinal cord levels, where the fibers reach the posterior (ie, dorsal) surface of the spinal cord. Fibers from the precentral motor cortex terminate mostly in laminae VII and IX, while fibers from Brodmann areas 1, 2, 3, and 5 terminate mostly in the posterior horn.
Fibers from the anterior CST (ie, bundle of Turk) descend uncrossed in the spinal cord, close to the anterior median fissure. The anterior CST is more developed in humans and apes than in other animals. Most fibers cross at the cervical levels, terminating bilaterally on neurons at lamina VII. It mainly modulates motor neurons that innervate neck and arm muscles.
The anterolateral CST is composed of small-diameter fibers, which run uncrossed in the lateral funiculus, more ventral than the lateral CST. They terminate in the posterior horn and intermediate gray matter.
Tracts originating in the brainstem
In addition to the CST, other tracts, originating in the brainstem, descend in the spinal cord. They are involved mainly in the control of postural tone.
A tectospinal tract arises from neurons in the deep layers of the superior colliculus, crosses around the periaqueductal gray matter, and is incorporated in the medial longitudinal fasciculus at the medulla. It descends in the anterior funiculus, near the anterior median fissure, only through cervical levels. Fibers terminate in laminae VII, VIII, and parts of VI, synapsing with interneurons. They mediate reflex postural movements in response to visual and, possibly, auditory stimuli.
A rubrospinal tract arises from magnocellular neurons (at least in primates) in the red nucleus and crosses at the ventral tegmental decussation. Stimulation of the red nucleus leads to excitation of contralateral flexor alpha motor neurons and inhibition of extensor alpha motor neurons. Fibers are organized somatotopically and terminate in the lateral half of laminae V, VI, and dorsal/central VII. Fibers mainly control muscle tone of flexor muscle groups.
Vestibulospinal tracts arise from the lateral vestibular nucleus (ie, Deiter nucleus) and descend bilaterally in the anterior part of the lateral funiculus. Fibers are organized somatotopically and terminate in laminae IX, VII, and VIII, mainly in the cervical and lower lumbar segments. They facilitate spinal cord reflexes and muscle tone. Interruption of this tract eliminates decerebration. Excitatory vestibulospinal input is present at rest and during locomotion. Fibers originating in the medial vestibular nucleus project medially in the medial longitudinal fasciculus and continue in the anterior funiculus. They modulate cervical motor neurons.
Two reticulospinal tracts arise in the pontine and medullary tegmenti. The pontine reticulospinal tract has its origin in the nucleus reticularis pontis caudalis and oralis. It descends almost completely ipsilaterally in the medial part of the anterior funiculus. It mainly produces monosynaptic and polysynaptic excitation of axial (more strongly) and limb muscles.
The medial longitudinal fasciculi run in the posterior part of the anterior funiculus and originate at different brainstem levels. They form a well-defined tract only in the cervical spinal cord but descend until the sacral regions. They inhibit upper cervical motor neurons and regulate head position. Fastigiospinal fibers cross the midline and project to cervical spinal cord levels, descending in the ventral part of the lateral funiculus. Their significance in motor control is largely unknown.
Posterior (ie, dorsal funiculi) columns convey 3 different types of sensation: (1) proprioception, or position sense, for which the sensory receptors are the muscle spindles and Golgi tendon organs; (2) vibratory sense, for which the receptor is the Pacinian corpuscle; and (3) discriminative touch, for which the receptor is the Meissner corpuscle. This information is carried by large, myelinated (ie, A alpha or type Ia) fibers in the sensory nerves. Some evidence indicates a possible role for the dorsal columns in visceral pain transmission. See the image below.[1]
 Spinal cord cross-section, detailed anatomy.
Spinal cord cross-section, detailed anatomy.
The cell bodies of the first-order unipolar neurons lie in the dorsal root ganglia just outside the cord parenchyma. Impulses enter the cord and are carried ipsilaterally. Fibers travel rostrally in the dorsal columns to synapse in the nucleus gracilis and nucleus cuneatus in the caudal medulla.
The fasciculus gracilis lies medial to the cuneatus in the posterior cord and subserves leg sensation. The fasciculus cuneatus lies lateral to the gracilis in the posterior cord and subserves arm sensation.
Second-order neurons contribute to the arcuate fasciculus. They decussate and subsequently ascend in the contralateral medial lemniscus to synapse in the ventroposterolateral (VPL) nucleus of the thalamus.
Third-order neurons travel in the posterior part of the posterior limb of the internal capsule to terminate in the primary and secondary somatosensory cortex (ie, postcentral gyrus and/or Brodmann areas 1-3).
The lateral spinothalamic tract lies in the ventrolateral cord and carries pain, temperature, and crude touch sensation. Smaller, unipolar neurons in the dorsal root ganglia are the first-order neuron for this tract. Impulses from naked nerve endings travel in small, thinly myelinated (ie, A delta and/or type II) and unmyelinated (ie, C or type III) fibers, which funnel into the dorsolateral fasciculus (ie, tract of Lissauer). Each axon bifurcates into ascending and descending branches, which extend for 1-2 segments and then give off collateral branches, which synapse in the ipsilateral dorsal horn (laminae I-VI).
Neurons located at lamina II (ie, substantia gelatinosa) seem to modulate the function of laminae III and IV, altering transmission from primary to secondary sensory systems. They receive projections from the brainstem reticular formation: periventricular and periaqueductal gray and nucleus raphe magnus.
The cell bodies of the second neuron are located in the marginal nucleus (lamina I) and proper sensory nucleus. Axons of second-order neurons cross in the ventral white commissure (just anterior to the central canal). They then ascend in the contralateral lateral spinothalamic tract to synapse in the VPL thalamic nucleus.
Fibers of the spinothalamic tract are somatotopically organized: sacral fibers are located laterally; lumbar, thoracic, and cervical fibers join medially.
Third-order neurons located at the VPL give rise to axons that travel in the posterior part of the posterior limb of the internal capsule to terminate in the primary and secondary somatosensory cortex.
An anterior spinothalamic tract ascends in the anterior and anterolateral funiculi. It originates mostly in lamina VII. Its fibers project to the periaqueductal gray matter and intralaminar thalamic nuclei. It carries light touch impulses; when lesioned, little or no disturbance in function is produced. Its collaterals also synapse in the medullary reticular formation.
Spinoreticular fibers also synapse in nearby areas, giving rise to multisynaptic reticulothalamic projections, which activate multiple areas of the cerebral cortex. These medial thalamic fibers are involved with arousal, attention, and motivational and affective aspects of pain perception.
Dorsal spinocerebellar tracts lie in the lateral cord and run ipsilaterally toward the ipsilateral vermis of the anterior cerebellar lobe, entering through the inferior cerebellar peduncle. This tract arises from the nucleus dorsalis of Clarke, which forms a column of neurons in the medial part of lamina VII from C8 to L2. It receives afferents directly from the collaterals of the lumbosacral parts of the gracile tract. It carries nonconscious sensation of muscle position and tone from the lower extremities.
Similar impulses from the upper extremities run through the cuneate tract, synapsing directly into the accessory cuneate nucleus in the medulla. It then gives rise to the cuneocerebellar tract, which enters through the inferior cerebellar peduncle to reach the paravermis of the anterior cerebellar lobe.
A small, ventral spinocerebellar tract also exists in humans. It relays impulses about the status of the descending influences over the spinal cord motor neurons. Its neurons are scattered in the anterior horn and intermediate zone and decussate in the spinal cord. They enter the cerebellum through the superior cerebellar peduncle.
This system is responsible for integration of different spinal cord segments during complex movement performance. It includes 3 groups of intraspinal neurons, as follows:
Preganglionic sympathetic neurons are located in the intermediolateral cell column (lamina VII), which lies in the lateral aspect of the gray matter at levels T1-L3.
Preganglionic fibers pass through the ventral roots, spinal nerves, and white communicating rami, ending in the sympathetic paravertebral ganglia at different levels. They are cholinergic. Second-order neurons then reach the end organ and in most cases use norepinephrine as their neurotransmitter.
Sacral preganglionic neurons are located in and near the intermediolateral nucleus of S2-S4. They are cholinergic and emerge from the spinal cord, synapsing in the end organ ganglia. Postganglionic neurons are cholinergic and control defecation, urination, and erection.
The urogenital tract is innervated by 3 groups of peripheral nerves: sacral parasympathetic, lumbar sympathetic, and sacral somatic nerves.
Parasympathetic preganglionic neurons are located in the intermediolateral gray matter (laminae V-VII). Sacral parasympathetic pathways run through the pelvic nerves and are the major excitatory pathways to the urinary bladder.
Sympathetic preganglionic neurons are located in the medial (lamina X) and lateral gray matter (laminae V-VII) of the rostral lumbar cord. Thoracolumbar sympathetic pathways come from the lumbar/sacral sympathetic ganglia; they inhibit the detrusor (beta mediated) and excite the base of the bladder and urethra (alpha mediated). Most importantly, they modulate the function of the parasympathetic ganglia (alpha-2 leads to inhibition and alpha-1 to facilitation).
Somatic efferent pathways innervate the urethral striated muscles and originate from a circumscribed lateral ventral horn region, known as Onuf's (or Onufrowicz') nucleus.
A alpha and C afferent pathways initiate micturition. A alpha fibers exhibit graded response to passive distension, while C fibers have a much higher threshold, being activated by inflammation and noxious stimuli. Fullness of the bladder is detected by receptors in the bladder wall, which send impulses through the sacral parasympathetic nerves. Impulses reach the cortex through the spinothalamic tracts.
Sensation that micturition is imminent arises from receptors located at the bladder trigone and ascends in the dorsal column system.
Urine is stored when the external urethral sphincter muscle (somatic) and the internal urethral sphincter muscle (sympathetic) are contracted and the detrusor muscle and sacral parasympathetic activity are inhibited through sympathetic mediation.
Sympathetic integrity is not essential for the performance of micturition. However, experimental evidence suggests that sympathetic input causes tonic inhibitory input to the bladder and excitatory input to the urethra. During micturition, descending pathways originating from the pontine micturition center inhibit external urethral sphincter activity, inhibit sympathetic outflow (inhibition of the vesicosympathetic reflex), activate parasympathetic outflow to the bladder, and activate parasympathetic outflow to the urethra.
For patient education information, see Bladder Control Problems.
Parasympathetic pathways arising from the sacral spinal cord innervate the erectile tissue in the penis and clitoris; smooth muscle and glandular tissue in the prostate, urethra, seminal vesicles, vagina, and uterus; and blood vessels and secretory epithelia in various structures. The most studied function has been penile erection, starting with the observation of Eckhard in 1863 that stimulation of the pelvic nerves leads to penile erection in several species. Penile erection is secondary to vasodilation of the penile blood vessels, with increased flow to the cavernous tissue. Nitric oxide (NO) is the principal mediator of penile erection in humans.
The spinal cord is supplied by descending branches of the vertebral arteries (ie, anterior spinal arteries) and multiple radicular arteries derived from segmental vessels.
Paired anterior spinal arteries unite to form a single descending vessel (ie, anterior spinal artery), which enters the anterior median fissure of the spinal cord and supplies the anterior two thirds of the cord. It also supplies midline rami to the lower medulla. Like the basilar artery, it has smaller penetrating and circumferential branches.
Two posterior spinal arteries each supply the ipsilateral posterior one sixth of the cord (or combined, the posterior one third). They receive varied contribution from the posterior radicular arteries and form 2 longitudinal plexiform channels near the dorsal root entry zone.
Radicular arteries are derived from segmental vessels (eg, ascending cervical, deep cervical, intercostal, lumbar, and sacral arteries) that pass the intervertebral foramina and give rise to anterior and posterior radicular arteries. Segmental radicular arteries supply blood to the roots, and segmental radiculospinal arteries supply the roots and the cord. Usually a few large, segmental radiculospinal arteries are noted, including the artery of Adamkiewicz (or artery of the lumbar enlargement), which is larger than the others; it usually originates between T9 and T12 (in 75% of cases) and supplies the lower one third of the cord.
Where 2 anterior radicular arteries reach the same level of the spinal cord, a diamond-shaped arterial configuration develops. The distance between radicular arteries is greatest in the thoracic spinal segments; thus, occlusion of 1 thoracic radicular artery may seriously compromise the circulation. Therefore, the upper thoracic (T1-4) and L1 segments are particularly vulnerable to vascular insults.
Veins draining the spinal cord have a distribution similar to that of the arteries. Anterior longitudinal trunks consist of anteromedian and anterolateral veins. Sulcal veins drain the anteromedian portions of the spinal cord. Anterolateral regions of the spinal cord drain into anterolateral veins. Posterior, longitudinal venous trunks drain the posterior funiculi. The internal vertebral venous plexuses (ie, epidural venous plexuses) are located between the dura mater and the vertebral periosteum and consist of 2 or more anterior and posterior, longitudinal venous channels that are interconnected at many levels from the clivus to the sacral region.
At each intervertebral space are connections with thoracic, abdominal, and intercostal veins and external vertebral venous plexuses. These spinal veins have no valves, and blood passes directly into the systemic venous system. The continuity of this venous plexus with the prostatic plexus is probably the path along which prostatic neoplastic cells metastasize.
Developments in magnetic resonance angiography with fast contrast-enhanced techniques have enabled noninvasive imaging of normal and abnormal vessels of the spinal cord, including visualization of the Adamkiewicz artery and differentiation of arteries and veins.[2] These techniques will likely improve the diagnosis of several conditions associated with abnormalities of the arterial and venous spinal cord supply.
The classic syndromes of spinal cord injury are described here. In most instances, however, incomplete forms are far more common.
In the acute phase, the classic syndrome of complete spinal cord transection at the high cervical level consists of the following:
This constellation of symptoms is called spinal shock.[3] Horner syndrome (ie, ipsilateral ptosis, miosis, and anhydrosis) is also present with higher lesions because of interruption of the descending sympathetic pathways originating from the hypothalamus.
Patients experience problems with temperature regulation because of the sympathetic impairment, which leads to hypothermia.
Lower cervical level injury spares the respiratory muscles. High thoracic lesions lead to paraparesis instead of quadriparesis, but autonomic symptoms are still marked. In lower thoracic and lumbar lesions, hypotension is not present but urinary and bowel retention are.
In the subacute phase, the flaccidity of spinal shock is replaced by the return of intrinsic activity of spinal neurons, and spasticity develops. This usually happens in humans within 3 weeks of injury. However, the spinal shock phase may be prolonged by other medical complications, such as infections.
Quadriplegia and sensory loss below the level of injury persist, but spinal reflexes return. Because modulation from supraspinal centers is lost, hyperreflexia with increased tone and extensor plantar responses are noted. At any given level, with more extensive involvement of the anterior horn, flaccidity with loss of reflex activity and atrophy is present in a lower motor neuron pattern (as is common in diseases such as poliomyelitis).
Autonomic hyperreflexia
Autonomic hyperreflexia is also present in the subacute phase. Usually, the initial hypotension after high lesions resolves, although orthostatic hypotension persists. For lesions above the lumbosacral centers for bladder control, the initial urinary retention is replaced by development of an automatic spastic bladder. Lower lesions lead to permanent atonic bladder (ie, lower motor neuron pattern).[4]
Autonomic hyperreflexia in this phase is characterized by massive firing of sympathetic neurons after distention, stimulation, or manipulation of the bladder and bowels. Cutaneous stimulation with painful or cold stimuli can also lead to massive sympathetic firing.
This is a life-threatening condition, because blood pressure may increase as high as 300 mm Hg, leading to intracerebral hemorrhage, confusional states, and death. A response to the massive sympathetic discharge is generated at the brainstem level. However, interruption of descending projections to the spinal cord can prevent inhibition of the spinal cord sympathetic centers, which continue to fire inappropriately until the stimulus is removed. A vagal inhibitory reflex to the heart is generated, which leads to bradycardia and worsening symptoms.
Anterior cord syndrome is typically observed with anterior spinal artery infarction and results in paralysis below the level of the lesion, with loss of pain and temperature sensation below the level of the lesion and relative sparing of touch, vibration, and position sense (because the posterior columns receive their primary blood supply from the posterior spinal arteries).
Central cord syndrome is observed most often in syringomyelia, hydromyelia, and trauma. Hemorrhage and intramedullary tumors, such as central canal ependymoma, are other causes. Because central cord syndrome is more common in the cervical cord, the arms are often weak, with preservation of strength in the legs ("man-in-a-barrel syndrome"). Considerable recovery is common. This syndrome is associated with variable sensory and reflex deficits; often the most affected sensory modalities are pain and temperature, because the lateral spinothalamic tract fibers cross just ventral to the central canal. This is sometimes referred to as dissociated sensory loss and is often present in a capelike distribution.
Lateral extension can result in ipsilateral Horner syndrome (because of involvement of the ciliospinal center), kyphoscoliosis (because of involvement of dorsomedian and ventromedian motor nuclei supplying the paraspinal muscles), and spastic paralysis (because of corticospinal tract involvement). Dorsal extension can result in ipsilateral position sense and vibratory loss due to involvement of the dorsal column.
Brown-Séquard syndrome may be considered equivalent to a hemicordectomy. Ipsilateral paralysis, loss of vibration and position sense below the level of the lesion, hyperreflexia, and an extensor toe sign all are noted. Ipsilateral segmental anesthesia is also observed at the lesion level. Loss of pain and temperature is observed contralaterally below the level of the lesion (beginning perhaps 2-3 segments below). Brown-Séquard syndrome is most common after trauma. However, the full spectrum of this syndrome is rare.
Patients with lesions affecting only the cauda equina can present with a polyradiculopathy in the lumbosacral area, with pain, radicular sensory changes, asymmetrical lower motor neuron–type leg weakness, and sphincter dysfunction. This may be difficult to distinguish from plexus or nerve involvement. Lesions affecting only the conus medullaris cause early disturbance of bowel and bladder functions. Syndromes due to involvement of the lower spinal cord, cauda equina, or conus have received more attention with advances in neuroimaging, especially in developing countries where helmintic infections may lead to spinal cord schistosomiasis.[5, 6]
Patients with radicular involvement present with dermatomal sensory changes with dorsal root involvement and with myotomal weakness with ventral root involvement. In general, radicular pain (eg, root distribution or shooting pain) increases with increased intraspinal pressure (eg, coughing, sneezing, any Valsalva maneuver).
Copyright © www.orthopaedics.win Bone Health All Rights Reserved