

An ideal anticoagulant should have the following characteristics:
These criteria are often difficult to achieve. Each of the anticoagulant agents available today incorporates some of these characteristics, but no single agent combines all these attributes.
Current research on anticoagulants involves investigations into drugs that act on various phases of the coagulation cascade. For convenience, the process can be arbitrarily divided into 3 phases: the initiation phase, the propagation phase, and the thrombin activity phase.[1]
See the image below.
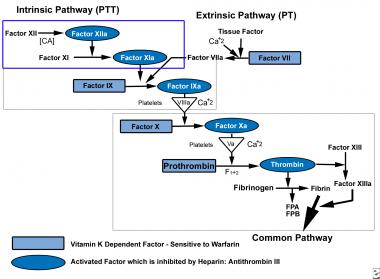 Coagulation pathway.
Coagulation pathway.
Go to Deep Venous Thrombosis and Pulmonary Embolism for more complete information on these topics.
NextDrugs under investigation that act in the initiation phase include tissue factor pathway inhibitors (TFPIs) and nematode anticoagulant peptide (NAPc2). These drugs act through inhibition of the factor VIIa/tissue factor complex.
Numerous new synthetic, direct or indirect antithrombin-dependent inhibitors of factor Xa are being tested. These have similarities to the currently approved fondaparinux. Phase II dose-finding trials in which the metapentasaccharide idraparinux was administered subcutaneously once each week to prevent the development of secondary venous thromboembolism have been completed. A second class of orally active direct factor Xa inhibitors, which includes razaxaban, is also undergoing clinical phase II trials.
Rivaroxaban (Xarelto) is a factor Xa inhibitor. The drug was approved by the FDA in July 2011 and is indicated for prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE) in patients undergoing knee or hip arthroplasty.
Large phase III clinical trials have been published describing the oral anticoagulant rivaroxaban for the prevention of thromboembolism following total knee or total hip arthroplasty. Rivaroxaban is administered once daily and, compared with subcutaneous enoxaparin,[2] has shown significant superiority in preventing DVT, nonfatal PE, and death, following arthroplastic surgery.[3, 4, 5, 6]
Drugs that act on the third stage of the coagulation cascade, the thrombin activity phase, include the direct thrombin inhibitors. These drugs specifically inactivate thrombin and are independent of antithrombin III.[7]
Included in the direct thrombin inhibitor group are the hirudins and their derivatives made by recombinant DNA techniques. Originally, hirudin was isolated from leech salivary gland tissue. The new drugs include bivalirudin (Angiomax) and lepirudin.
A randomized, multicenter, double-blind study of hirudin versus heparin in patients with total hip replacements demonstrated that DVT and proximal DVT rates were decreased substantially in the hirudin group.
Another class of drugs acting at the thrombin activity phase of the coagulation cascade includes the noncovalent inhibitor argatroban, which is a carboxylic acid derivative that has been approved for use in the treatment of heparin-induced thrombocytopenia (HIT).
Copyright © www.orthopaedics.win Bone Health All Rights Reserved