

In the times of Hippocrates and Galen, distal radius fractures (DRFs) were thought to be wrist dislocations. Pouteau first varied from this tradition when he described a variety of forearm fractures in the French literature, including a DRF. As a result, DRFs are termed Pouteau fractures in the French-speaking world. However, politics and communications being what they were, the English-speaking world did not recognize the Pouteau description.
The Irish surgeon Abraham Colles described DRFs in the 1814 volume of the Edinburgh Medical Surgical Journal. Although his description was based on clinical examination alone (because radiography had not yet been invented), it is quite accurate, and it is Colles' name that is most often associated with this fracture in the English-speaking world. Colles stated, "One consolation only remains, that the limb will at some remote period again enjoy perfect freedom in all of its motions and be completely exempt from pain...." This claim that all DRFs, despite displacement, will fare well has been a source of criticism.
Over time, other eponyms have been added to the various subclassifications of DRFs, such as the Smith fracture, Barton fracture, and volar Barton fracture. The fractures are also referred to as various stages of classification systems, such as a Melone IV or an AO (ie, Arbeitsgemeinschaft für Osteosynthese [Association for the Study of Osteosynthesis]) C3 fracture, or are referred to the region of the fracture (eg, a radial styloid or a lunate facet fracture), or have a historical explanation (chauffeur's fracture, so called because a chauffeur sustained this injury when he tried to crank-start a car and it backfired).
In current practice, as a result of greater knowledge of the varieties of fracture configurations, eponyms tend to be avoided, and a direct description of the fracture is preferred. The term DRF properly covers all fractures of the distal articular and metaphyseal areas. Although all classification systems have serious problems, there is general agreement on the meaning of at least some of the classification terms (eg, Melone IV or AO C3 fracture), and these terms do add some degree of specificity and understanding to the generic designation DRF. (See Presentation, Classification.)
The ultimate aim of treatment is to restore each patient to his or her prior level of functioning. The specific goals, therefore, will not be the same in all patients. For example, a 21-year-old athlete wants to resume competition, but an 82-year-old person usually only wants to return to activities of daily living (ADLs).[1, 2]
Because goals differ, treatment options differ as well. In addition, because people now remain active until an older age, the definition of "prior level of functioning" is changing. For example, a 92-year-old patient who was being treated in the emergency department (ED) had only one concern when conversing with his physician: how soon he could return to playing golf (he had a tournament the next week). Treatment goals, therefore, must be tailored to each patient. Specifically, treatment should be determined not by age but by activity level.
NextTreatment of a DRF depends on a solid understanding of the anatomy of the radius.
On the volar surface of the radius (see the image below), the large lunate facet is seen on the left, projecting out from the surface of the radius. The volar radial tuberosity is at the right margin of the bone. The surface is covered with the pronator quadratus (PQ). The cortical bone is quite thick and is strong, even in osteoporotic patients.
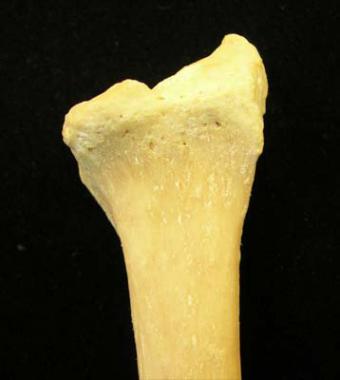 Volar surface of radius.
Volar surface of radius.
On the dorsal surface of the radius (see the image below), the Lister tubercle is seen in the center. This bone is a thin cortical shell, with little structural strength.
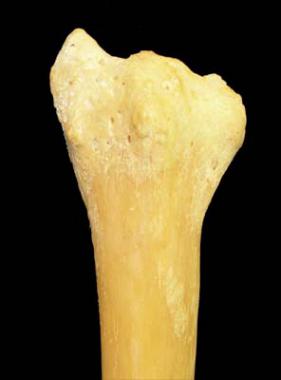 Dorsal surface of radius.
Dorsal surface of radius.
The radial surface of the radius is shown in the image below.
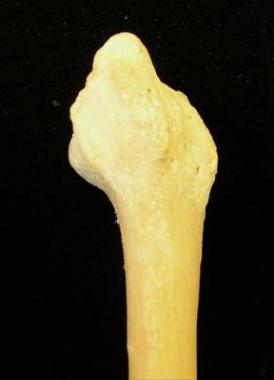 Radial surface of radius.
Radial surface of radius.
The ulnar surface of the radius, with the sigmoid notch for articulating with the ulna, is shown in the image below.
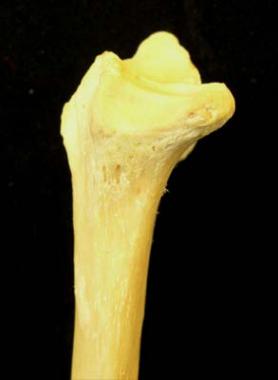 Ulnar surface of radius.
Ulnar surface of radius.
On the distal articular surface of the radius (see the image below), the scaphoid facet is to the right, and the lunate facet is to the left. This bone is the strongest of all the surfaces, and even if it is osteoporotic, it is quite strong.
 Distal surface of radius.
Distal surface of radius.
A normal posteroanterior (PA) radiograph of the radius is shown in the image below. The ulna is generally within (plus or minus) 2 mm of the radius.
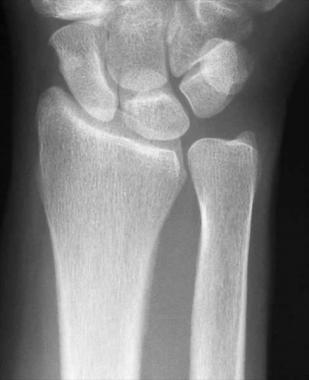 Posteroanterior radiograph of normal wrist.
Posteroanterior radiograph of normal wrist.
A normal lateral radiograph is shown in the image below. Note that the center of the lunate facet overlies the volar surface of the bone.
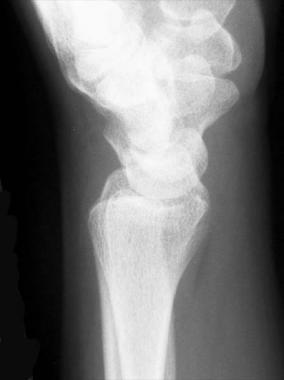 Lateral radiograph of normal wrist.
Lateral radiograph of normal wrist.
Several anatomic landmarks are important for the volar approach to the radius (see the image below).
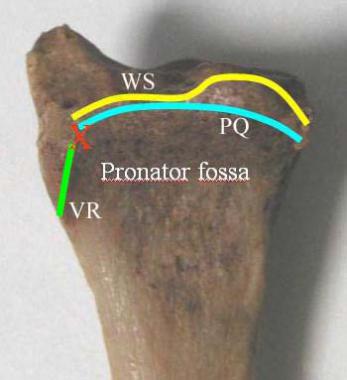 Volar anatomic landmarks important for volar approach. Region marked "pronator fossa" is covered by pronator quadratus (PQ) . It extends distally to PQ line, marked in blue. Watershed line (WS) marks highest crest (most volarly projecting surface) of radius. Red X marks volar radial tuberosity, which lies just off PQ. It is usually not dissected and therefore usually not seen, but it is easily palpable clinically. VR = volar radial ridge.
Volar anatomic landmarks important for volar approach. Region marked "pronator fossa" is covered by pronator quadratus (PQ) . It extends distally to PQ line, marked in blue. Watershed line (WS) marks highest crest (most volarly projecting surface) of radius. Red X marks volar radial tuberosity, which lies just off PQ. It is usually not dissected and therefore usually not seen, but it is easily palpable clinically. VR = volar radial ridge.
The pathophysiology of a fracture is rather obvious: more load is imparted to a bone than the bone can sustain. Osteoporotic bone can break with very low impact. However, the patient should always be questioned regarding the circumstances of the injury, especially if he or she is older. Heart attacks or transient ischemic attacks can cause a DRF and should not be overlooked.
In addition, more problems may be involved with the injury than just the fracture. A useful perspective is that a DRF is a soft-tissue injury surrounding a broken bone, and the immediacy of the radiographic diagnosis should not distract the surgeon from carefully assessing systemic issues or forearm soft-tissue issues.[3]
Younger patients have stronger bone, and thus, more energy is required to create a fracture in these individuals. Motorcycle accidents, falls from a height, and similar situations are common causes of a DRF. Trauma is the leading cause of death in the 15- to 24-year-old age group, and this is also reflected in the incidence of lesser traumas.
Older patients have much weaker bones and can sustain a DRF from simply falling on an outstretched hand in a ground-level fall. An increasing awareness of osteoporosis has led to these injuries being termed fragility fractures, with the implication that a workup for osteoporosis should be a standard part of treatment. As the population lives longer, the frequency of this type of fracture will increase.
DRFs are among the most common types of fracture, and many authors state that they are the single most common type. DRFs have a bimodal distribution, with a peak in younger persons (aged 18-25 years) and a second peak in older persons (aged >65 years). The mechanism of injury is unique to each group, with high-energy injuries being more common in the younger group and low-energy injuries being more common in the older group.
Unresolved treatment controversies notwithstanding, most patients can resume their previous level of activity, including competitive sports. Although many cases have been reported in which return to function was not limited by malunion or complications, patients are, in general, living longer and continuing to be active longer than in previous generations, and this places demands on the distal radius that were not seen previously. Consequently, even with apparently good care, some patients are unable to resume their prior level of functioning.
All treatment approaches have a percentage of poor results, with decreased supination, prominent ulnar heads, ligamentous problems, distal radioulnar problems (usually instability), and degenerative joint disease being common problems. These are the cases that prompt researchers to continue to refine the techniques and devices.[4]
Patients, however, want more concrete prognostic statements. To this end, the following may be stated:
It should be kept in mind that these are just general guidelines, and great variation exists among specific cases and specific physicians.
The long-term prognosis for a properly treated DRF is good, even with an intra-articular fracture. If the articular surface is not comminuted and can be reconstructed, osteoarthritis is rare. Wrist range of motion will continue to increase, and wrist tenderness with forceful use will continue to decrease even beyond 2 years.
Clinical Presentation
Copyright © www.orthopaedics.win Bone Health All Rights Reserved