

Surgery for Dupuytren contracture generally should be performed on an affected metacarpophalangeal (MCP) joint if the contracture is 30° or greater. Such contractures most likely cause some debilitation for the patient. Usually, a limited fasciectomy of the pretendinous cord is sufficient to establish normal function in the MCP joint.
The evaluation of a proximal interphalangeal (PIP) joint in Dupuytren disease is different from that of an MCP joint, and the prognoses differ as well. In PIP joint contractures, one should clearly define the method to be used in surgery and discuss with the patient his or her expectations and occupation, as well as activities the patient engages in that may require the use of his or her hands.
Complications occur most often in patients who require total fasciectomy because of severe disease; McFarlane and McGrouther reported a complication rate of as high as 17-19%.[1] During surgery, complications may include severing of the digital nerves, most often the neurovascular bundle; the inadvertent creation of a buttonhole through the skin flaps during their separation between the skin and the fascia; and circulatory compromise secondary to trauma to the digital arteries.
Postoperative complications include loss of flexion, hematoma, skin sloughing, infection, edema, and reflex sympathetic dystrophy. The most common postoperative PIP-joint complication is loss of flexion, which occurs in 6% of patients.
The triad of hematoma, infection, and skin loss occurs in 3% of patients. Hematomas most often form in the palm, and they may be prevented by meticulous hemostasis, by removal of the tourniquet before the wound is closed, and by rapid evacuation of hematomas, which prevents necrosis of tissue and skin and decreases the risk of infection. Elevation of the hand can prevent postoperative edema.
Reflex sympathetic dystrophy more commonly occurs in patients with extensive fasciectomies and, thus, more aggressive disease. This idiopathic pain syndrome, which often occurs 3-4 weeks after the surgery, consists of pain, edema, stiffness, and vasomotor disturbances. Reflex sympathetic dystrophy occurs in 5% of patients, affecting 3% of men and 7% of women; treatment includes sympathetic blockade for symptomatic relief.
The patient will likely judge his or her own result subjectively, with the perception of functional improvement as an endpoint. Andrew found that MCP contracture invariably was corrected (86% excellent), while good results for PIP contracture were less frequent (40% excellent result in middle, ring fingers; 20% in small fingers).[2] Excellent results in the correction of secondary contracture of the distal interphalangeal (DIP) joint occurred in approximately 50% of cases. (See the images below.)
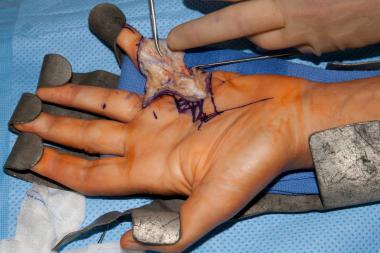 Dissection of spiral cord in the hand of a patient with Dupuytren disease. Image courtesy of Lawrence Yeung, MD.
Dissection of spiral cord in the hand of a patient with Dupuytren disease. Image courtesy of Lawrence Yeung, MD.
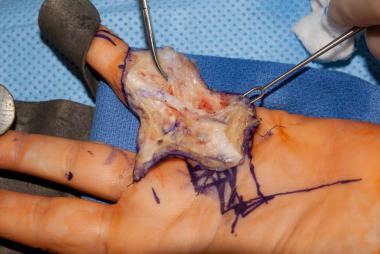 Dissection of the spiral cord in a patient with Dupuytren disease. Image courtesy of Lawrence Yeung, MD.
Dissection of the spiral cord in a patient with Dupuytren disease. Image courtesy of Lawrence Yeung, MD.
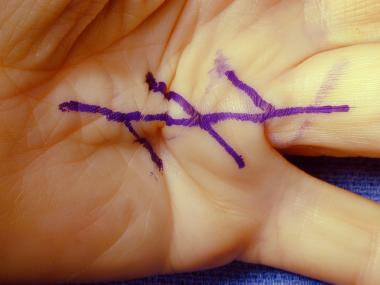 Visible cord characteristic of Dupuytren disease with planned markings for surgical release.
Visible cord characteristic of Dupuytren disease with planned markings for surgical release.
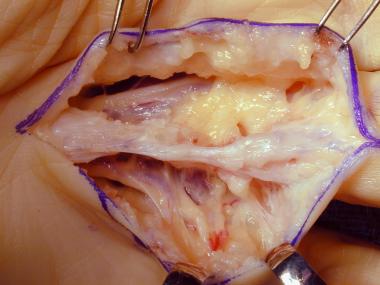 Dissection of a diseased cord.
Next
Dissection of a diseased cord.
Next
Indications for surgery depend on the patient’s requirements for hand function, the patient’s age, the severity of the contracture, and the joint or joints involved. Generally, surgery is not necessary until contracture occurs.[3]
MCP flexion contracture of 30° or more is an accepted criterion for surgical correction.[3, 4] At this point, flexion becomes functionally significant for most people; even in the ulnar digits where there is some carpometacarpal (CMC) extension range. MCP joint flexion contracture of up to 60o has been easily correctable.[5]
Proximal interphalangeal (PIP) flexion contracture of any degree constitutes an indication for surgery. A flexion contracture of the PIP joint quickly becomes more difficult to correct because of shortening of collateral ligaments and fibrosis and adherence of periarticular structures such as the volar plate.
Adequate correction may require joint release after the resection of contracted, diseased tissue. Shortening of neurovascular bundles can also limit extension after release in long-standing PIP contracture. Because of these technical challenges, complete correction is less common, and persistence of joint flexion contracture is more likely.[5, 2]
Neurovascular compromise due to Dupuytren disease is an indication for intervention. Secondary involvement of periarticular structures may also require correction.
Thumb flexion deformities are well-tolerated and less functionally significant; therefore, they are less urgently corrected. However, significant first web space contracture involving the thumb warrants surgical intervention.[5]
Some believe that early surgical intervention is more likely than delayed surgery to yield excellent results, while others believe that, with natural disease progression, radical prophylactic palmar clearance potentiates the need for revisions.[6, 7] Surgical techniques for Dupuytren disease include closed (or percutaneous) and open fasciotomy, regional (or limited) palmar fasciectomy, and radical (or total) fasciectomy.
Two important considerations are adequate release of longitudinal tension and the management of involved skin. Other important decisions involve selection of the incisions required and any special method of wound closure employed. The patient’s age and functional requirements and the potential for recurrent disease also impact decisions on the extent of surgery.
Total fasciectomy prevents recurrence because the entire diseased fascia is removed, along with the central, lateral, spiral, natatory, and retrovascular cords, as well as any normal fascia that may later be affected.
As stated above, a limited fasciectomy of the pretendinous cord is usually sufficient to establish normal function in the MCP joint. McFarlane favors use of a regional fasciectomy of the pretendinous cord to prevent recurrence of Dupuytren contracture.[1] For longitudinal incisions, Z-plasties or multiple Y-to-V advancements may adequately close the wound. A transverse incision may be necessary for more extensive disease; in such cases, a defect may require a full-thickness skin graft or necessitate the wound to heal secondarily.[8]
Given the difficulty of correcting severe disease, fasciectomy is indicated for any amount of PIP joint contracture. Unfortunately, recurrence is common. The procedures of choice in the PIP joint are dermatofasciectomy or total fasciectomy.
In a study by Donaldson et al, the extent of a patient’s preoperative deformity was found to be a significant predictor of complete intraoperative correction in Dupuytren contracture. In addition, the extent of preoperative deformity and intraoperative correction were significant predictors of loss of surgical correction after surgery. The investigators prospectively studied 52 patients who underwent primary fasciectomy for Dupuytren disease, to determine whether preoperative contracture and the amount of intraoperative correction can predict postoperative outcome.[9]
In the study, 42 MCP joints were treated, of which 41 achieved full intraoperative correction; 37 had full correction at 6 months' follow-up. Of the 58 PIP joints treated surgically, full intraoperative correction was obtained in 35, and 13 had complete correction at 6 months' follow-up.
In another study, Villani et al found that full-thickness skin grafts prevented recurrence of Dupuytren contracture in 20 out of 23 hands after more than 8 years' follow-up. Of the 18 patients who underwent dermofasciectomy and skin grafting, 13 received skin grafts on 1 hand, and 5 patients received grafts on both hands. The authors noted that because recurrence is difficult to predict, primary skin grafting remains controversial and that indications for the skin-grafting procedure are more definite once recurrence has taken place.[10, 11]
The surgeon and/or patient may choose either regional (local, median, or ulnar nerve block) or general anesthesia for the procedure. Regional anesthesia performed more proximally decreases tourniquet-related discomfort. Hurst uses Marcaine (bupivacaine HCl; Cook-Waite, Cambridge, Ontario, Canada) without epinephrine for its longer duration of nerve blockage. (Note that regional anesthesia should not be used if the patient has any of the following conditions: coagulopathy, psychosis, peculiar and/or unstable personality, or progressive neurologic disease.)
In closed fasciotomy for Dupuytren disease, a limiting cord of diseased, superficial fascia is incised via an overlying skin incision. This technique can be successful in MCP contractures but is not as useful for PIP contracture, since more than 1 cord is usually present. Closed fasciotomy presents some risk of neurovascular injury.
One indication described is to facilitate hygiene in a debilitated elderly patient who has contractures that keep the fingernails in contact with the palmar skin or produce secondary wounds. Closed fasciotomy has also been used as an initial stage in very severe contracture to facilitate further release.
Duthie and Chesney concluded that closed fasciotomy is a useful procedure for patients who may be "unsuitable for local fasciectomy." The investigators reported on a nonselected series of 160 patients treated with closed fasciotomy, with a 10-year follow-up for the 51% of patients remaining alive.[12] Although contracture progressed in most patients, 34% required no further surgery, while the mean time to fasciectomy for the remaining 66% was 5 years. A 4% complication rate was described for this outpatient surgery, performed under local anesthesia or nerve block.[12, 13]
Open fasciotomy allows direct visualization of neurovascular structures. The offending cord is divided at a point not immediately underlying the skin incision. Fasciotomies are usually most successful for MCP flexion contracture. This procedure can be performed under local anesthesia and recovery is rapid; however, the recurrence rate is high. Open fasciotomy is usually reserved for patients who cannot tolerate a more extensive procedure.
Segmental aponeurectomy of Moermans[14, 15] is a procedure that is intermediate between simple fasciotomy and limited fasciectomy. Segments (1cm in length) of fascia are excised through C-shaped incisions. Moermans claims that a Dupuytren cord can resolve once the tension across it is diminished. A prospective study performed by Andrew demonstrated a recurrence rate comparable to that of other techniques, but with fewer complications.[16] This is an outpatient procedure.
Percutaneous needle fasciotomy (PNF), adopted by a group of French rheumatologists and repopularized by Foucher and other European surgeons, is a minimally invasive treatment that is usually performed as an office procedure under local anesthesia.[17, 18] It involves multiple puncture sites and sectioning of the Dupuytren cord using the bevel of a needle.
In study of 211 older patients, (average age 65y), 1 digital nerve injury, no infections, and no tendon injuries were found with needle "aponeurotomy."[19] However, recurrence (58%) and disease activity (69%) were high at the 3-year follow-up.
Foucher et al believed this technique to be ideal for the elderly patient with a bowing cord and a predominant MCP joint contracture.[19] Limitations of fasciotomy in treating digital disease and PIP contracture were again noted.
Contraindications to PNF include infiltrating disease, rapid recurrence in a young patient, inaccessible multiple cords, chronic digital disease, and postsurgical recurrence in the digits.[19]
Van Rijssen et al[20] compared PNF with limited fasciectomy, with short-term follow up, and found less discomfort, quick recovery, and better immediate hand function in the PNF group. PNF demonstrated an improvement of 63% in passive extension deficit and no significant complications. While fasciectomy produced more improvement in contracture, particularly in more advanced cases, the major complication rate was 5%. The authors also noted the need to avoid applying PNF in a zone at the junction of the palm and the base of the finger where the neurovascular bundle may be displaced superficially and toward the midline and be more vulnerable to injury.
Van Rijssen and colleagues concluded from another study that PNF was a good treatment alternative to limited fasciectomy in patients with a total passive extension deficit of 90o or less.
This is the most commonly performed procedure for Dupuytren disease. In regional, or limited, fasciectomy, only the diseased parts of the superficial fascial aponeurosis are excised. These include, for example, pretendinous cords and involved natatory ligaments in the palm, as well as the visibly affected structures in the fingers. Although Dupuytren disease may recur or progress by extension in the nonoperated areas of the hand, good results have been obtained, with acceptable complication rates.[7, 21]
In another study, Hueston concluded that regional fasciectomy does not prevent recurrence but does allow correction of deformity, with more rapid recovery of hand function. In his report, Hueston described complications in 96 operated hands, finding hematoma in 7.5%, problematic or persistent edema in 15.5%, digital nerve injury in 2%, skin necrosis in 2%, and wound infection in 1%.[7] "Functional recovery" was delayed beyond 6 weeks in 15.5%. He found the lower rate of hematomas to be less than half of that reported in radical fasciectomy.At 2-year follow-up in this series, 27 patients were found to have "extension" and 12 patients were found to have true recurrence of diseased tissue.
Radical, or total, surgery was thought by McIndoe and Beare to "cure" Dupuytren disease.[6] They sought to eliminate recurrent Dupuytren disease through complete removal of the palmar aponeurosis and natatory ligaments, based on the idea that Dupuytren nodules cannot form if no remnant of palmar fascia is present. In the digits, all diseased cords and tissue that may be affected are excised.
McIndoe and Beare[6] reported satisfactory results in over 200 cases with an extended or total palmar fasciectomy utilizing a transverse palmar incision with separate Z-plasty incisions (used, when necessary, in the digits). The authors, who employed hypotensive anesthesia and drains, reported that skin grafting was practically never necessary. Specific data on complications was not provided.
While McIndoe and Beare believed that small hematomas would drain spontaneously, others report hematoma formation with subsequent swelling and stiffness (as well as infection) to be a formidable problem with this procedure.[7]
Unfortunately, recurrent disease was not eliminated by the more extensive surgery. Hueston found a nearly equal recurrence rate at 5- to 15-year follow-up in a comparison of limited fasciectomy and more radical procedures.[7] He reserved radical fasciectomy for those few patients with extensive and diffuse involvement of the entire palm in Dupuytren disease. He found this approach to be necessary in roughly 10% of his patients.
McCash reduced the incidence of hematoma by leaving his transverse palmar incision open for closure by secondary intention (open palm technique).[22] The McCash technique is most often used when diffuse involvement of the entire palm dictates extended or radical fasciectomy. A delayed skin graft can be employed for closure of the palmar wound.
Hueston encountered a 28% overall rate of recurrence following surgical treatment of Dupuytren disease. Early and aggressive (or repeated) recurrence was seen in younger patients. Hueston found that full-thickness skin grafts appeared to "arrest" this process. He theorized that the skin flaps overlying fasciectomy were the (extrinsic) source of recurrent Dupuytren tissue rather than unresected elements of palmar fascia left after fasciectomy.[23, 24]
These observations led him to employ dermofasciectomy for recurrent disease, particularly in the digits. In dermofasciectomy, diseased fascia and the overlying skin are excised completely, and full-thickness skin grafting is applied for closure. Later reports of McCann and Logan suggested the dermis as a possible source for myofibroblasts causing recurrent disease.[25]
Tonkin reported that dermofasciectomy with skin grafting prevented recurrent Dupuytren disease without compromising hand function, suggesting it as a prophylactic approach in young patients with Dupuytren diathesis.[26]
Logan recommended dermofasciectomy as the first line of treatment for recurrent digital Dupuytren disease but found that it did not prevent recurrence in all cases.[27] He also noted that the immobilization required for the associated skin grafts interfered with early postoperative rehabilitation.
McFarlane has criticized this approach because it may not address the presence of diseased retrovascular tissue and suggests that the exposed flexor tendon sheath is unfavorable as a graft bed. He felt that it was usually possible to separate the diseased fascia from the overlying skin.
Other authors have confirmed recurrent Dupuytren disease following this procedure.[28, 29] Armstrong et al found a recurrence rate of 11.6% in 103 patients undergoing dermofasciectomy, but they still advanced it as the best method for control of "diffuse Dupuytren disease with involvement of the skin."[30] They stated the possibility that dermofasciectomy was not popular because of concerns about the success of the skin grafting required.
Successful use of bone distraction and tissue expansion techniques has led to the use of distraction devices in conjunction with fasciectomies. According to Messina, this technique of gradual passive extension has allowed salvage of severely contracted digits.[31, 32, 33, 34]
The application of continuous passive extension was used to elongate the contracted palmar fascia. Authors described reorganization of the once densely packed collagen fibers in the cords of Dupuytren disease into a parallel, ribbonlike appearance.[32]
In 1994, another device for PIP extension, referred to as the Proximal Interphalangeal Skeletal Traction Extender, was introduced by Hodgkinson for preoperative outpatient use.[35] Authors believe this device makes adjacent tissues more available and decreases PIP flexion contracture, facilitating successful surgery.
Correction of the PIP joint is a more difficult technical problem in Dupuytren disease. If complete extension is not obtained by careful digital fasciectomy, the options are either to rely on postoperative therapy and splinting or to perform some form of volar PIP joint release. PIP joint release is usually employed when the flexion contracture is greater than 30o. The flexor sheath can be incised, and the lateral proximal attachment of the volar plate (so-called checkrein ligaments) released, as necessary.
However, if these maneuvers do not achieve full PIP extension, some recommend further joint capsulotomy, as described by Curtis.[36] His stepwise approach involved sequential release of accessory collateral ligaments followed by release of the proper collateral ligaments on one side of the joint at a time until full joint extension was achieved or all structures had been released. Others have cautioned that the correction achieved at surgery would not be maintained and that aggressive capsulotomy of the PIP joint is likely to result in permanent loss of flexion range, which is more limiting than a mild flexion contracture.[37]
In 1979, Watson and associates examined 115 checkrein releases and found full intraoperative extension in 110 joints, with additional release necessary in only 5 joints.[38] They concluded that releasing the accessory collateral or proper collateral ligaments is almost never required with successful checkrein excision.
McFarlane and Botz discouraged the use of capsulotomy in patients with chronic PIP contracture if correction to 40º of flexion or less could be obtained.[37]
A study by Weinzweig et al of 42 involved PIP joints in 28 patients demonstrated no advantage to capsuloligamentous release compared with fasciectomy alone.[39] The authors also felt that stretching or adherence of the extensor mechanism with prolonged flexion contracture could render it ineffective, contributing to late return of flexion deformity.
Alternatives for severe PIP joint contracture include arthroplasty (including implant arthroplasty) and arthrodesis. The shortening concomitant with arthroplasty (or arthrodesis) results in improvement of the contracture. While PIP arthrodesis establishes a desired functional angle at the joint, it further limits function.
Amputation is rarely necessary in digital disease. It is usually performed in elderly patients with a severely contracted fifth digit following thorough surgeon-patient discussion and realistic analysis of attainable function.
Hyperextension of the DIP joint usually occurs secondary to long-standing PIP joint contracture, with foreshortening of the Landsmeer ligament (oblique retinacular ligament); the DIP joint itself remains normal. If DIP joint deformity is passively correctable, it usually resolves with correction of PIP contracture. If it is not passively correctable, division of Landsmeer ligaments usually corrects the deformity. Severe fibrosis of dorsal skin related to knuckle pads can also limit DIP joint flexion.
Various incisions can facilitate exposure during surgery for Dupuytren disease.[40] Incisions can be transverse, longitudinal, or combined, depending on the pattern of involvement.
For a single digit, a midline volar incision closed with multiple Z-plasties can be employed. Some authors believe that a midline digital incision is least likely to expose a neurovascular bundle to injury. Alternatively, a volar zig-zag incision or V-Y plasty can be used.[41] Overall, digital Z-plasty incisions have been shown to give excellent exposure and functionally stable scars.[6]
For palmar disease with multiple MCP flexion contractures, a transverse incision at the level of distal palmar crease can be made. This can be joined to longitudinal digital incisions if necessary. Part of the palmar incision(s) can be left open, as in McCash’s open technique. Alternatives for palmar disease include palmar V-Y plasty.
Safe dissection during surgery for Dupuytren disease is enhanced by the use of loupe magnification. Sharp dissection is usually employed in separation of skin from the underlying diseased fascia. Dissection of the skin from the underlying fascia may leave very thin skin flaps, particularly in the digits.
If skin grafts are employed in the digits, they should extend from midlateral line to midlateral line across the digit.
In a severely involved digit, the neurovascular bundle may be most easily located distally. Dissecting retrovascular disease is important. Awareness of possible displacement of the neurovascular structures is essential.
Proximal division of the pretendinous cord may facilitate dissection by allowing finger extension and abduction.
In planning reoperative surgery for Dupuytren disease, if clinical evaluation suggests that the digital nerve has been severed, one should assume that the related digital artery has also been severed and should confirm adequate contralateral circulation to that digit.
Excision of skin (and fascia) leaves a wound that requires additional coverage. Although McIndoe and Beare believed that grafts were practically unnecessary,[6, 42] they reported a lower recurrence rate of Dupuytren disease when grafts or flaps were placed in flexion creases. Generally, the latter believes that skin replacement should be reserved for young patients who have an active diathesis, postfasciectomy recurrence, or a rapid progression of skin fixation and deformity.[43] Skin grafting has also been used in conjunction with the open-palm technique of McCash.
Good results with a technique of limited palmar fasciectomy with skin grafting have been reported.[44]
Reports have not confirmed either a lower rate of recurrence or better functional results when skin grafting is combined with other procedures.[45]
Generally, authors recommending digital dermofasciectomy prefer full-thickness skin grafting to split-thickness skin grafting, because of increased wound contraction beneath the latter. Ipsilateral inner-arm donor sites can be used for skin grafting, while distant donor sites, including the distal lower extremity, have been suggested for improved cosmesis.[30]
The successful use of skin grafts requires a protective dressing and precludes early or vigorous interphalangeal joint movement.[46]
Local flap wound closure (beyond Z-plasty) has rarely been used. An L-shaped skin flap, called the Jacobsen flap, was developed as a modification of the McCash technique by Tripoli and Merle.[47] Upon flap transposition, a more limited 15mm palmar skin defect is left to heal by secondary intention. The authors reported satisfactory correction of contracture and a low complication rate in 98 cases using this technique.
Free microvascular transfer of a circumflex scapular artery perforator flap was reported for coverage of a very large palmar defect after radical dermofasciectomy for disabling recurrent Dupuytren disease.[48] Following multiple revisions and extensive hand rehabilitation, flexion deformities were significantly improved and satisfactory function was obtained.
Proper postoperative care is essential for a successful surgical outcome. The protocol includes splinting in extension and an exercise regimen with a therapist for the institution of range-of-motion exercises within the first week after surgery. Patients who undergo PIP joint surgery require 6 weeks of continual splinting, including splinting at night, and may require bracing for as long as 3 months overall to minimize secondary scar contractures.[49, 50]
Since surgery for Dupuytren disease is most often performed on an outpatient basis, close follow-up in the early postoperative period is recommended. Early motion is encouraged. Some surgeons feel that routine, formal, supervised hand therapy at an early stage of healing is important for functional rehabilitation. Many use intermittent static extension splinting for more resistant contractures. As wound healing progresses, the patient can be encouraged to use the hand in activities of daily living, and more vigorous passive range-of-motion exercises can be employed.
Tourniquet release and meticulous hemostasis prior to wound closure is recommended. Adequate drainage, such as an open area in a palmar surgical site, has been beneficial.
Skin flaps can fail for many reasons, but underlying hematoma is a frequent problem. The skin may be very thin after dissection from the underlying fascia. In this situation, if skin viability is in doubt, a preemptive skin graft may be a better option. Potential donor sites can be identified preoperatively.
Infection usually follows hematoma, skin loss, or both. If contracture prevents adequate skin preparation, a fasciotomy can be performed as a preliminary measure before definitive fasciectomy.
Dissection in a fasciectomy is similar to neurolysis, ie, the involved neurovascular bundles must be dissected free along the entire course of the surgical wound. The area of greatest risk is adjacent to the web space over the base of the proximal phalanx. Awareness of the displacement of the neurovascular bundle to the midline by a spiral cord is important. Surgeons should be prepared for appropriate repair of divided nerves and arteries.
Loss of flexion range: This is a common late complication. Active and passive preoperative range of motion should be recorded. Active flexion exercises should be part of early postoperative care. Maintenance of flexion range is often neglected in the effort to regain full extension. Schneider reported a 41% loss of flexion range after surgery for palmar disease.[3]
Several authors have warned that reflex sympathetic dystrophy (RSD) is a significant problem following surgery for Dupuytren disease.[51, 52, 53] Luck reported that features of RSD were observed with increased frequency after surgery for Dupuytren disease.[54] It is at least 5 times more common in women than in men with Dupuytren disease.
Local hyperalgesia, possibly due to digital nerve injury and neuroma formation, can be problematic.[54]
Recurrence (ie, Dupuytren tissue forming in the area of resection) and recurrence of flexion deformity with disease extension (ie, Dupuytren tissue appearing outside the area of resection) are believed to be separate entities.[55] Recurrence is much more likely in a young patient with a strong family history and knuckle pads.[7] A recurrence rate ranging between 26% and 80% has been reported.
In his evaluation of 224 patients postfasciectomy, Hueston concluded that recurrence is rare after 2 years postoperatively. He also concluded that recurrence is less frequent in older patients but is an early postoperative event in younger patients with Dupuytren diathesis, with some patients requiring multiple reoperations.[23, 24] The incidence of recurrence has been decreased, but not completely eliminated, with skin replacement techniques.
Copyright © www.orthopaedics.win Bone Health All Rights Reserved