

Osteoporosis (see the image below) is the most common metabolic bone disease in the United States and can result in devastating physical, psychosocial, and economic consequences. It is often overlooked and undertreated, however, in large part because it is clinically silent before manifesting as fracture.
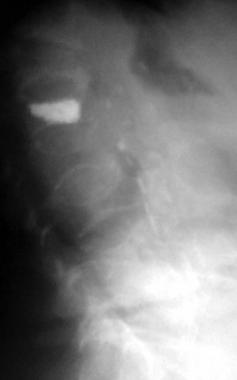 Osteoporosis. Lateral radiograph demonstrates multiple osteoporotic vertebral compression fractures. Kyphoplasty has been performed at one level.
Osteoporosis. Lateral radiograph demonstrates multiple osteoporotic vertebral compression fractures. Kyphoplasty has been performed at one level.
See Menopause: Changes and Challenges, a Critical Images slideshow, to help identify comorbidities and diseases in the postmenopausal population.
Osteoporosis generally does not become clinically apparent until a fracture occurs. Two thirds of vertebral fractures are painless. Typical findings in patients with painful vertebral fractures may include the following:
Patients who have sustained a hip fracture may experience the following:
On physical examination, patients with vertebral compression fractures may demonstrate the following:
Patients with hip fractures may demonstrate the following:
Patients with Colles fractures may have the following:
Patients with pubic and sacral fractures may have the following:
Balance difficulties may be evident, especially in patients with an altered center of gravity from severe kyphosis.[1] Patients may have difficulty performing tandem gait and performing single limb stance.
See Clinical Presentation for more detail.
Baseline laboratory studies include the following:
Bone mineral density (BMD) measurement is recommended in the following patients[2] :
Dual-energy x-ray absorptiometry (DXA) is currently the criterion standard for the evaluation of BMD.[3, 4] Peripheral DXA is used to measure BMD at the wrist; it may be most useful in identifying patients at very low fracture risk who require no further workup.
DXA provides the patient’s T-score, which is the BMD value compared with that of control subjects who are at their peak BMD.[5, 6, 7, 8] World Health Organization (WHO) criteria define a normal T-score value as within 1 standard deviation (SD) of the mean BMD value in a healthy young adult. Values lying farther from the mean are stratified as follows[7] :
DXA also provides the patient’s Z-score, which reflects a value compared with that of persons matched for age and sex. Z-scores adjusted for ethnicity or race should be used in the following patients:
Z-score values of –2.0 SD or lower are defined as "below the expected range for age" and those above –2.0 SD as "within the expected range for age." The diagnosis of osteoporosis in these groups should not be based on densitometric criteria alone.
Quantitative calcaneal ultrasonography offers the following benefits[9] :
However, no diagnostic criteria based on quantitative ultrasonography or a combination of quantitative ultrasonography and DXA have been defined.
The National Osteoporosis Foundation (NOF) recommends vertebral imaging for the following patients[2] :
Vertebral imaging is also recommended for postmenopausal women and men age 50 and older with the following specific risk factors:
If bone density testing is not available, vertebral imaging may be considered based on age alone.
Other plain radiography features and recommendations are as follows:
See Workup for more detail.
Lifestyle modification for prevention of osteoporotic fractures includes the following[10] :
The NOF recommends that pharmacologic therapy should be reserved for postmenopausal women and men aged 50 years or older who present with the following[2] :
Guidelines from the American Association of Clinical Endocrinologists include the following recommendations for choosing drugs to treat osteoporosis[11] :
Guidelines from the American College of Rheumatology for the treatment of glucocorticoid- induced osteoporosis include the following[12] :
Medical care also includes the identification and treatment of potentially treatable underlying causes of osteoporosis such as hyperparathyroidism and hyperthyroidism. Surgical care in selected patients may include vertebroplasty and kyphoplasty, which are minimally invasive spine procedures used for the management of painful osteoporotic vertebral compression fractures.
See Treatment and Medication for more detail.
NextOsteoporosis, a chronic, progressive disease of multifactorial etiology (see Etiology), is the most common metabolic bone disease in the United States. It has been most frequently recognized in elderly white women, although it does occur in both sexes, all races, and all age groups. Screening at-risk populations is essential (see Workup).
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility.[13] The disease often does not become clinically apparent until a fracture occurs (see the following image).
 Osteoporosis. Lateral radiograph demonstrates multiple osteoporotic vertebral compression fractures. Kyphoplasty has been performed at one level.
Osteoporosis. Lateral radiograph demonstrates multiple osteoporotic vertebral compression fractures. Kyphoplasty has been performed at one level.
Osteoporosis represents an increasingly serious health and economic problem in the United States and around the world.[14] Many individuals, male and female, experience pain, disability, and diminished quality of life as a result of having this condition.
Despite the adverse effects of osteoporosis, it is a condition that is often overlooked and undertreated, in large part because it is so often clinically silent before manifesting in the form of fracture. For example, a Gallup survey performed by the National Osteoporosis Foundation revealed that 86% of women with osteoporosis had never discussed its prevention with their physicians.[15] Failure to identify at-risk patients, to educate them, and to implement preventive measures may lead to tragic consequences.
Medical care includes calcium, vitamin D, and antiresorptive agents such as bisphosphonates, the selective estrogen receptor modulator (SERM) raloxifene, calcitonin, and denosumab. One anabolic agent, teriparatide (see Medication), is available as well. Surgical care includes vertebroplasty and kyphoplasty (see Treatment).
Osteoporosis is a preventable disease that can result in devastating physical, psychosocial, and economic consequences. Prevention and recognition of the secondary causes of osteoporosis are first-line measures to lessen the impact of this condition (see the images below).
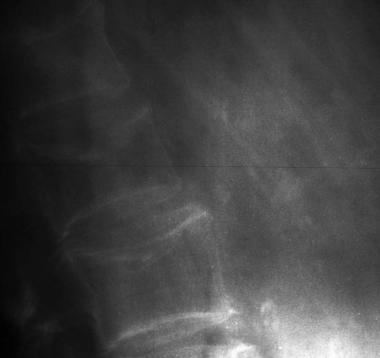 Osteoporosis of the spine. Observe the considerable reduction in overall vertebral bone density and note the lateral wedge fracture of L2.
Osteoporosis of the spine. Observe the considerable reduction in overall vertebral bone density and note the lateral wedge fracture of L2.
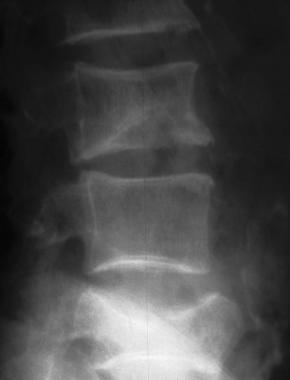 Osteoporosis of the spine. Note the lateral wedge fracture in L3 and the central burst fracture in L5. The patient had suffered a recent fall.
Osteoporosis of the spine. Note the lateral wedge fracture in L3 and the central burst fracture in L5. The patient had suffered a recent fall.
Bone mineral density (BMD) in a patient is related to peak bone mass and, subsequently, bone loss. Whereas the T-score is the patient’s bone density compared with the BMD of control subjects who are at their peak BMD, the Z-score reflects a bone density compared with that of patients matched for age and sex.[5, 6, 7, 8]
The World Health Organization’s (WHO) definitions of osteoporosis based on BMD measurements in white women are summarized in Table 1, below.[7, 8] For each standard deviation (SD) reduction in BMD, the relative fracture risk is increased 1.5-3 times.
The WHO definition applies to postmenopausal women and men aged 50 years or older. Although these definitions are necessary to establish the prevalence of osteoporosis, they should not be used as the sole determinant of treatment decisions. This diagnostic classification should not be applied to premenopausal women, men younger than 50 years, or children.
Table 1. WHO Definition of Osteoporosis Based on BMD Measurements by DXA (Open Table in a new window)
Definition Bone Mass Density Measurement T-Score Normal BMD within 1 SD of the mean bone density for young adult women T-score ≥ –1 Low bone mass (osteopenia) BMD 1–2.5 SD below the mean for young-adult women T-score between –1 and –2.5 Osteoporosis BMD ≥2.5 SD below the normal mean for young-adult women T-score ≤ –2.5 Severe or “established” osteoporosis BMD ≥2.5 SD below the normal mean for young-adult women in a patient who has already experienced ≥1 fractures T-score ≤ –2.5 (with fragility fracture[s]) Sources:Z-scores should be used in premenopausal women, men younger than 50 years, and children. Z-scores adjusted for ethnicity or race should be used, with Z-scores of –2.0 or lower defined as "below the expected range for age" and with Z-scores above –2.0 being defined as "within the expected range for age." The diagnosis of osteoporosis in these groups should not be based on densitometric criteria alone.
For more information, see Pediatric Osteoporosis, as well as Osteoporosis in Solid Organ Transplantation, Bone Markers in Osteoporosis, and Nonoperative Treatment of Osteoporotic Compression Fractures.
It is increasingly being recognized that multiple pathogenetic mechanisms interact in the development of the osteoporotic state. Understanding the pathogenesis of osteoporosis starts with knowing how bone formation and remodeling occur.
Bone is continually remodeled throughout our lives in response to microtrauma. Bone remodeling occurs at discrete sites within the skeleton and proceeds in an orderly fashion, and bone resorption is always followed by bone formation, a phenomenon referred to as coupling.
Dense cortical bone and spongy trabecular or cancellous bone differ in their architecture but are similar in molecular composition. Both types of bone have an extracellular matrix with mineralized and nonmineralized components. The composition and architecture of the extracellular matrix is what imparts mechanical properties to bone. Bone strength is determined by collagenous proteins (tensile strength) and mineralized osteoid (compressive strength).[18] The greater the concentration of calcium, the greater the compressive strength. In adults, approximately 25% of trabecular bone is resorbed and replaced each year, compared with only 3% of cortical bone.
Osteoclasts, derived from hematopoietic precursors, are responsible for bone resorption, whereas osteoblasts, from mesenchymal cells, are responsible for bone formation (see the images below). The 2 types of cells are dependent on each other for production and linked in the process of bone remodeling. Osteoblasts not only secrete and mineralize osteoid but also appear to control the bone resorption carried out by osteoclasts. Osteocytes, which are terminally differentiated osteoblasts embedded in mineralized bone, direct the timing and location of bone remodeling. In osteoporosis, the coupling mechanism between osteoclasts and osteoblasts is thought to be unable to keep up with the constant microtrauma to trabecular bone. Osteoclasts require weeks to resorb bone, whereas osteoblasts need months to produce new bone. Therefore, any process that increases the rate of bone remodeling results in net bone loss over time.[19]
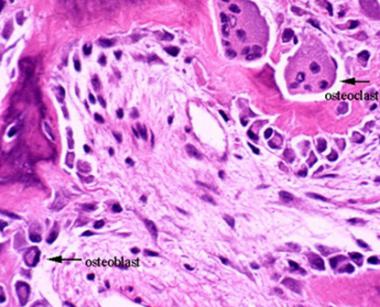 This image depicts bone remodeling with osteoclasts resorbing one side of a bony trabecula and osteoblasts depositing new bone on the other side.
This image depicts bone remodeling with osteoclasts resorbing one side of a bony trabecula and osteoblasts depositing new bone on the other side.
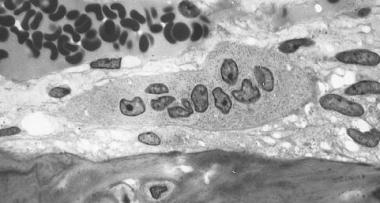 Osteoclast, with bone below it. This image shows typical distinguishing characteristics of an osteoclast: a large cell with multiple nuclei and a "foamy" cytosol.
Osteoclast, with bone below it. This image shows typical distinguishing characteristics of an osteoclast: a large cell with multiple nuclei and a "foamy" cytosol.
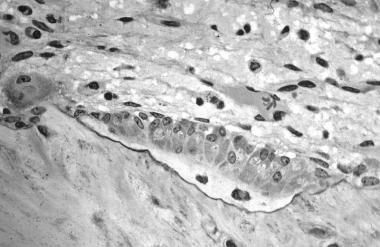 In this image, several osteoblasts display a prominent Golgi apparatus and are actively synthesizing osteoid. Two osteocytes can also be seen.
In this image, several osteoblasts display a prominent Golgi apparatus and are actively synthesizing osteoid. Two osteocytes can also be seen.
Furthermore, in periods of rapid remodeling (eg, after menopause), bone is at an increased risk for fracture because the newly produced bone is less densely mineralized, the resorption sites are temporarily unfilled, and the isomerization and maturation of collagen are impaired.[20]
The receptor activator of nuclear factor-kappa B ligand (RANKL)/receptor activator of nuclear factor-kappa B (RANK)/osteoprotegerin (OPG) system is the final common pathway for bone resorption. Osteoblasts and activated T cells in the bone marrow produce the RANKL cytokine. RANKL binds to RANK expressed by osteoclasts and osteoclast precursors to promote osteoclast differentiation. OPG is a soluble decoy receptor that inhibits RANK-RANKL by binding and sequestering RANKL.
Bone mass peaks around the third decade of life and slowly decreases afterward. A failure to attain optimal bone strength by this point is one factor that contributes to osteoporosis, which explains why some young postmenopausal women have low bone mineral density (BMD) and why some others have osteoporosis. Therefore, nutrition and physical activity are important during growth and development. Nevertheless, hereditary factors play the principal role in determining an individual's peak bone strength. In fact, genetics account for up to 80% of the variance in peak bone mass between individuals.[10, 21]
The hallmark of osteoporosis is a reduction in skeletal mass caused by an imbalance between bone resorption and bone formation. Under physiologic conditions, bone formation and resorption are in a fair balance. A change in either—that is, increased bone resorption or decreased bone formation—may result in osteoporosis.
Osteoporosis can be caused both by a failure to build bone and reach peak bone mass as a young adult and by bone loss later in life. Accelerated bone loss can be affected by hormonal status, as occurs in perimenopausal women; can impact elderly men and women; and can be secondary to various disease states and medications.
Aging and loss of gonadal function are the 2 most important factors contributing to the development of osteoporosis. Studies have shown that bone loss in women accelerates rapidly in the first years after menopause. The lack of gonadal hormones is thought to up-regulate osteoclast progenitor cells. Estrogen deficiency leads to increased expression of RANKL by osteoblasts and decreased release of OPG; increased RANKL results in recruitment of higher numbers of preosteoclasts as well as increased activity, vigor, and lifespan of mature osteoclasts.
Estrogen deficiency
Estrogen deficiency not only accelerates bone loss in postmenopausal women but also plays a role in bone loss in men. Estrogen deficiency can lead to excessive bone resorption accompanied by inadequate bone formation. Osteoblasts, osteocytes, and osteoclasts all express estrogen receptors. In addition, estrogen affects bones indirectly through cytokines and local growth factors. The estrogen-replete state may enhance osteoclast apoptosis via increased production of transforming growth factor (TGF)–beta.
In the absence of estrogen, T cells promote osteoclast recruitment, differentiation, and prolonged survival via IL-1, IL-6, and tumor necrosis factor (TNF)–alpha. A murine study, in which either the mice's ovaries were removed or sham operations were performed, found that IL-6 and granulocyte-macrophage CFU levels were much higher in the ovariectomized mice.[22] This finding provided evidence that estrogen inhibits IL-6 secretion and that IL-6 contributes to the recruitment of osteoclasts from the monocyte cell line, thus contributing to osteoporosis.
IL-1 has also been shown to be involved in the production of osteoclasts. The production of IL-1 is increased in bone marrow mononuclear cells from ovariectomized rats. Administering IL-1 receptor antagonist to these animals prevents the late stages of bone loss induced by the loss of ovarian function, but it does not prevent the early stages of bone loss. The increase in the IL-1 in the bone marrow does not appear to be a triggered event but, rather, a result of removal of the inhibitory effect of sex steroids on IL-6 and other genes directly regulated by sex steroids.
T cells also inhibit osteoblast differentiation and activity and cause premature apoptosis of osteoblasts through cytokines such as IL-7. Finally, estrogen deficiency sensitizes bone to the effects of parathyroid hormone (PTH).
Aging
In contrast to postmenopausal bone loss, which is associated with excessive osteoclast activity, the bone loss that accompanies aging is associated with a progressive decline in the supply of osteoblasts in proportion to the demand. This demand is ultimately determined by the frequency with which new multicellular units are created and new cycles of remodeling are initiated.
After the third decade of life, bone resorption exceeds bone formation and leads to osteopenia and, in severe situations, osteoporosis. Women lose 30-40% of their cortical bone and 50% of their trabecular bone over their lifetime, as opposed to men, who lose 15-20% of their cortical bone and 25-30% of trabecular bone.
Calcium deficiency
Calcium, vitamin D, and PTH help maintain bone homeostasis. Insufficient dietary calcium or impaired intestinal absorption of calcium due to aging or disease can lead to secondary hyperparathyroidism. PTH is secreted in response to low serum calcium levels. It increases calcium resorption from bone, decreases renal calcium excretion, and increases renal production of 1,25-dihydroxyvitamin D (1,25[OH]2 D)—an active hormonal form of vitamin D that optimizes calcium and phosphorus absorption, inhibits PTH synthesis, and plays a minor role in bone resorption.
Vitamin D deficiency
Vitamin D deficiency can result in secondary hyperparathyroidism via decreased intestinal calcium absorption.
Osteoporotic fractures
Osteoporotic fractures represent the clinical significance of these derangements in bone. They can result both from low-energy trauma, such as falls from a sitting or standing position, and from high-energy trauma, such as a pedestrian struck in a motor vehicle accident. Fragility fractures, which occur secondary to low-energy trauma, are characteristic of osteoporosis.
Fractures occur when bones fall under excess stress. Nearly all hip fractures are related to falls.[23] The frequency and direction of falls can influence the likelihood and severity of fractures. The risk of falling may be amplified by neuromuscular impairment due to vitamin D deficiency with secondary hyperparathyroidism or corticosteroids.
Vertebral bodies are composed primarily of cancellous bone with interconnected horizontal and vertical trabeculae. Osteoporosis not only reduces bone mass in vertebrae but also decreases interconnectivity in their internal scaffolding.[18] Therefore, minor loads can lead to vertebral compression fractures.
An understanding of the biomechanics of bone provides greater appreciation as to why bone may be susceptible to an increased risk of fracture. When vertical loads are placed on bone, such as tibial and femoral metaphyses and vertebral bodies, a substantial amount of bony strength is derived from the horizontal trabecular cross-bracing system. This system of horizontal cross-bracing trabeculae assists in supporting the vertical elements, thus limiting lateral bowing and fractures that may occur with vertical loading.
Disruption of such trabecular connections is known to occur preferentially in patients with osteoporosis, particularly in postmenopausal women, making females more at risk than males for vertebral compression fractures (see the images below).
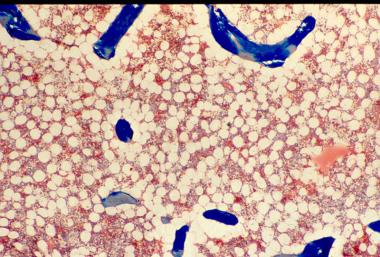 Osteoporosis is defined as a loss of bone mass below the threshold of fracture. This slide (methylmethacrylate embedded and stained with Masson's trichrome) demonstrates the loss of connected trabecular bone.
Osteoporosis is defined as a loss of bone mass below the threshold of fracture. This slide (methylmethacrylate embedded and stained with Masson's trichrome) demonstrates the loss of connected trabecular bone.
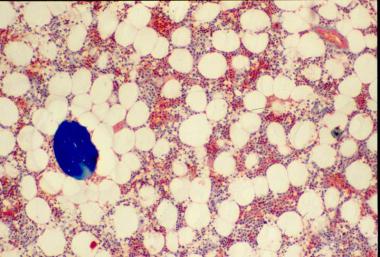 The bone loss of osteoporosis can be severe enough to create separate bone "buttons" with no connection to the surrounding bone. This easily leads to insufficiency fractures.
The bone loss of osteoporosis can be severe enough to create separate bone "buttons" with no connection to the surrounding bone. This easily leads to insufficiency fractures.
Rosen and Tenenhouse studied the unsupported trabeculae and their susceptibility to fracture within each vertebral body and found an extraordinarily high prevalence of trabecular fracture callus sites within vertebral bodies examined at autopsy, typically 200-450 healing or healed fractures per vertebral body.[24] These horizontal trabecular fractures are asymptomatic, and their accumulation reflects the impact of lost trabecular bone and greatly weakens the cancellous structure of the vertebral body.
The reason for preferential osteoclastic severance of horizontal trabeculae is unknown. Some authors have attributed this phenomenon to overaggressive osteoclastic resorption.
Osteoporosis versus osteomalacia
Osteoporosis may be confused with osteomalacia. The normal human skeleton is composed of a mineral component, calcium hydroxyapatite (60%), and organic material, mainly collagen (40%). In osteoporosis, the bones are porous and brittle, whereas in osteomalacia, the bones are soft. This difference in bone consistency is related to the mineral-to-organic material ratio. In osteoporosis, the mineral-to-collagen ratio is within the reference range, whereas in osteomalacia, the proportion of mineral composition is reduced relative to organic material content.
The Wnt signaling pathway and bone
The Wnt family is a highly conserved group of proteins that were initially studied in relationship with cancer initiation and progression due to their involvement in intercellular communication.[25] In the past decade, the Wnt signaling cascade has been recognized as a critical regulator of bone metabolism.
Wnt signaling plays a key role in the fate of mesenchymal stem cells (MSCs), which are the progenitor cells of mature bone-forming osteoblasts.[26] MSCs have the capability to differentiate into adipocytes, chondrocytes, neurons, and muscle cells, as well as into osteoblasts.[27] Certain Wnt signaling pathways promote the differentiation of MSCs along the osteoblast lineage. The emerging details about the specific molecules involved in the Wnt pathway have improved the understanding of bone metabolism and led to the development of new therapeutic targets for metabolic bone diseases.
Wnt signal activation may progress along one of three pathways, with the “canonical” pathway involving β-catenin being most relevant to bone metabolism. The canonical Wnt signaling pathway is initiated by the binding of a Wnt protein to an extracellular co-receptor complex consisting of “Frizzled” (Fr) and low density lipoprotein receptor–related protein–5 or –6 (LRP5, LRP6).[28] This activation recruits another protein, “Disheveled” (Dvl) to the intracellular segment of the Fz/Dvl co-receptor.[29] This is where β-catenin comes into play.
β-Catenin is an important intracellular signaling molecule and normally exists in a phosphorylated state targeted for ubiquination and subsequent degradation within intracellular lysosomes. Activation of the Wnt pathway leads to dephosphorylation and stabilization of intracellular β-catenin and rising cytosolic concentrations of β-catenin. As the concentration of β-catenin reaches a critical level, β-catenin travels to the nucleus, where it activates the transcription of Wnt target genes. Ultimately, canonical Wnt signaling inhibits the expression of transcription factors important in the differentiation of MSCs such as peroxisome proliferator-activated receptor gamma (PPAR-γ) and promotes survival of osteoblast lineage cells.[30]
Several human bone abnormalities have been linked to the Wnt pathway. For example, a single amino acid substitution in the LRP5 receptor gene has been associated with high bone mass phenotypes in humans; specifically, the mutant LRP5 receptor had an impaired interaction with the Wnt signal inhibitor Dickkopf-1 (Dkk-1).[31] Similarly, other missense mutations of LRP5 have been implicated in other high bone mass diseases such as Van Buchem disease and osteopetrosis.[32] Conversely, loss-of-function mutations of LRP5 have resulted in a rare but severe congenital osteoporosis in humans.[33]
There are also several antagonists to the Wnt pathway. Two of the most well-known are Dkk-1 and sclerostin (SOST). Dkk-1 is secreted by MSCs[34] and binds to LRP-5 and LRP-6,[35] thereby competitively inhibiting Wnt signaling. Interestingly, serum levels of Dkk-1 positively correlate with the extent of lytic bone lesions in patients with multiple myeloma.[36] Clinical trials of a monoclonal antibody to Dkk-1 are ongoing.[37]
Similarly, SOST, a product of osteocytes,[38] has also been found to antagonize the Wnt signaling pathway by binding to LRP5 and LRP6.[39] A SOST antibody is also undergoing clinical trials for treatment of metabolic bone disease.[40]
Additional factors and conditions
Endocrinologic conditions or medications that lead to bone loss (eg, glucocorticoids) can cause osteoporosis. Corticosteroids inhibit osteoblast function and enhance osteoblast apoptosis.[41] Polymorphisms of IL-1, IL-6 and TNF-alpha, as well as their receptors, have been found to influence bone mass.
Other factors implicated in the pathogenesis of osteoporosis include polymorphisms in the vitamin D receptor; alterations in insulin-like growth factor-1, bone morphogenic protein, prostaglandin E2, nitrous oxide, and leukotrienes; collagen abnormalities; and leptin-related adrenergic signaling.[19]
Prenatal and postnatal factors contribute to adult bone mass. In one study, the health of the mother in pregnancy, the infant’s birth weight, and the child’s weight at age 1 year were predictive of adult bone mass in the seventh decade for men and women.[42] It is postulated that growth in the first year of life programs growth hormone that is maintained into the seventh decade.[43] Larger babies and rapid growth in the first year of life predicted increased bone mass in adults aged 65-75 years.
Osteoporosis has been divided into several classifications according to etiology and localization in the skeleton. Osteoporosis is initially divided into localized and generalized categories, and these two main categories are further classified further into primary and secondary osteoporosis.
Postmenopausal osteoporosis (PMO) is primarily due to estrogen deficiency. Senile osteoporosis is primarily due to an aging skeleton and calcium deficiency.
Patients are said to have primary osteoporosis when a secondary cause of osteoporosis cannot be identified, including juvenile and idiopathic osteoporosis. Idiopathic osteoporosis can be further subdivided into postmenopausal (type I) and age-associated or senile (type II) osteoporosis, as described in Table 2, below.
Table 2. Types of Primary Osteoporosis (Open Table in a new window)
Type of Primary Osteoporosis Characteristics Juvenile osteoporosisSecondary osteoporosis occurs when an underlying disease, deficiency, or drug causes osteoporosis (see Table 3, below). Up to one third of postmenopausal women, as well as many men and premenopausal women, have a coexisting cause of bone loss,[44, 45] of which renal hypercalciuria is one of the most important secondary causes of osteoporosis and treatable with thiazide diuretics.[46]
Table 3. Causes of Secondary Osteoporosis in Adults (Open Table in a new window)
Cause Examples Genetic/congenitalRisk factors for osteoporosis, such as advanced age and reduced bone mineral density (BMD), have been established by virtue of their direct and strong relationship to the incidence of fractures; however, many other factors have been considered risk factors based on their relationship to BMD as a surrogate indicator of osteoporosis.
Risk factors for osteoporosis include the following[53, 54, 55] :
A potentially useful mnemonic for osteoporotic risk factors is OSTEOPOROSIS, as follows:
According to the National Osteoporosis Foundation (NOF), 9.9 million Americans have osteoporosis and an additional 43.1 million have low bone density. In the United States, two million fractures are attributed to osteoporosis annually, with 432,000 hospital admissions, 2.5 million medical office visits and approximately 180,000 nursing home admissions.[2]
Most studies assessing the prevalence and incidence of osteoporosis use the rate of fracture as a marker for the presence of this disorder, although BMD also relates to risk of disease and fracture. The risk of new vertebral fractures increases by a factor of 2-2.4 for each standard deviation (SD) decrease of BMD measurement. Women and men with metabolic disorders associated with secondary osteoporosis have a 2- to 3-fold higher risk of hip and vertebral fractures.
Globally, osteoporosis is by far the most common metabolic bone disease, estimated to affect over 200 million people worldwide.[58] An estimated 75 million people in Europe, the United States, and Japan have osteoporosis.[59]
Risk for osteoporosis increases with age as BMD declines. Senile osteoporosis is most common in persons aged 70 years or older. Secondary osteoporosis, however, can occur in persons of any age. Although bone loss in women begins slowly, it speeds up around the time of menopause, typically at about or after age 50 years. The frequency of postmenopausal osteoporosis is highest in women aged 50-70 years.
The number of osteoporotic fractures increases with age. Wrist fractures typically occur first, when individuals are aged approximately 50-59 years.
Vertebral fractures occur more often in the seventh decade of life. Jensen et al studied Danish women aged 70 years and found a 21% prevalence of vertebral fractures.[60] Melton et al reported that 27% of women in their study had evidence of vertebral fractures by age 65 years.[61]
Ninety percent of hip fractures occur in persons aged 50 years or older, occurring most often in the eighth decade of life.[62]
Women are at a significantly higher risk for osteoporosis. Half of all postmenopausal women will have an osteoporosis-related fracture during their lifetime; 25% of these women will develop a vertebral deformity, and 15% will experience a hip fracture.[63] Risk factors for hip fracture are similar in different ethnic groups.[64]
Men have a higher prevalence of secondary osteoporosis, with an estimated 45-60% of cases being a consequence of hypogonadism, alcoholism, or glucocorticoid excess.[50] Only 35-40% of osteoporosis diagnosed in men is considered primary in nature. Overall, osteoporosis has a female-to-male ratio of 4:1.
Fifty percent of all women and 21% of all men older than 50 years experience one or more osteoporosis-related fractures in their lifetime.[65] Eighty percent of hip fractures occur in women.[62] Women have a two-fold increase in the number of fractures resulting from nontraumatic causes, as compared with men of the same age.
Osteoporosis can occur in persons of all races and ethnicities. In general, however, whites (especially of northern European descent) and Asians are at increased risk. In particular, non-Hispanic white women and Asian women are at higher risk for osteoporosis. In the most recent government census, 178 million Chinese were over age 60 years in 2009, a number that the United Nations estimates may reach 437 million—one-third of the population—by 2050.[66]
These numbers suggest that approximately 50% of all hip fractures will occur in Asia in the next century. In fact, age-standardized incidence rates of fragility fractures, particularly of the hip and forearm, have been noted to be decreasing in the last decade across many countries, with the notable exception of Asia.[67]
Table 4, below, summarizes some osteoporosis prevalence statistics among racial/ethnic groups. Note that this disease is under-recognized and undertreated in white and black women. Relative to other racial/ethnic groups, the risk of developing osteoporosis is increasing fastest among Hispanic women.
Table 4. Prevalence of Osteoporosis Among Racial and Ethnic Groups (Open Table in a new window)
Race/Ethnicity Sex (age ≥50 y) % Estimated to have osteoporosis % Estimated to have low bone mass Non-Hispanic white; Asian Women 15.8 52.6 Men 3.9 36 Non-Hispanic black Women 7.7 36.2 Men 1.3 21.3 Hispanic Women 20.4 47.8 Men 5.9 38.3 Source: Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. Nov 2014;29(11):2520-6. [Medline].Melton et al reported that the prevalence of hip fractures is higher in white populations, regardless of geographic location.[68] Another study indicated that, in the United States and South Africa, the incidence of hip fractures was lower in black persons than in age-matched white persons. Cauley et al found that the absolute fracture incidence across bone mineral density (BMD) distribution was 30-40% lower in black women than in white women. This lower fracture risk was independent of BMD and other risk factors.[69]
The prognosis for osteoporosis is good if bone loss is detected in the early phases and proper intervention is undertaken. Patients can increase BMD and decrease fracture risk with the appropriate anti-osteoporotic medication. In addition, patients can decrease their risk of falls by participating in a multifaceted approach that includes rehabilitation and environmental modifications. Worsening of medical status can be prevented by providing appropriate pain management and, if indicated, orthotic devices.
Many individuals experience morbidity associated with the pain, disability, and diminished quality of life caused by osteoporosis-related fractures. According to a 2004 Surgeon General's report, osteoporosis and other bone diseases are responsible for about 1.5 million fractures per year. Osteoporosis-related fractures result in annual direct care expenditures of $12.2 billion to $17.9 billion.[70] In 2005, over 2 million osteoporosis-related fractures occurred in the United States.[71]
Osteoporosis is the leading cause of fractures in the elderly. Women aged 50 years have about a 50% lifetime fracture rate as a result of osteoporosis. Osteoporosis is associated with 80% of all the fractures in people aged 50 years or older.
If full recovery is not achieved, osteoporotic fractures may lead to chronic pain, disability, and, in some cases, death. This is particularly true of vertebral and hip fractures.
Vertebral fractures
Vertebral compression fractures (see the images below) are associated with increased morbidity and mortality rates. In addition, the impact of vertebral fractures increases as they increase in number. As posture worsens and kyphosis progresses, patients experience difficulty with balance, back pain, respiratory compromise, and an increased risk of pneumonia. Overall function declines, and patients may lose their ability to live independently.
 Osteoporosis. Lateral radiograph demonstrates multiple osteoporotic vertebral compression fractures. Kyphoplasty has been performed at one level.
Osteoporosis. Lateral radiograph demonstrates multiple osteoporotic vertebral compression fractures. Kyphoplasty has been performed at one level.
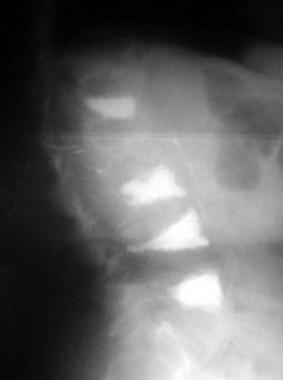 Osteoporosis. Lateral radiograph of the patient seen in the previous image following kyphoplasty performed at 3 additional levels.
Osteoporosis. Lateral radiograph of the patient seen in the previous image following kyphoplasty performed at 3 additional levels.
In one study, Cooper et al found that vertebral fractures increased the 5-year risk of mortality by 15%.[72] In a subsequent study, Kado et al[73] demonstrated that women with one or more fractures had a 1.23-fold increased age-adjusted mortality rate and that women with 5 or more vertebral fractures had a 2.3-fold increased age-adjusted mortality rate.
Furthermore, mortality rate was correlated with number of vertebral fractures, with 19 per 1000 woman-years in women with no fracture, versus 44 per 1000 woman-years in women with five or more fractures. Vertebral fractures were related to risk of subsequent cancer and pulmonary death, and severe kyphosis was further correlated with pulmonary deaths.
Only one third of people with radiographic vertebral fractures are diagnosed clinically.[74] Symptoms of vertebral fracture may include back pain, height loss, and disabling kyphosis. Compression deformities can lead to restrictive lung disease, abdominal pain, and early satiety.
Hip fractures
More than 250,000 hip fractures are attributed to osteoporosis each year. Like vertebral fractures, they are associated with significantly increased morbidity and mortality rates in men and women. In the year following hip fracture, excess mortality rates can be as high as 20%.[72, 75] Men have higher mortality rates following hip fracture than do women.
Patients with hip fractures incur decreased independence and a diminished quality of life. Of all patients with hip fracture, approximately 20% require long-term nursing care.[2] Among women who sustain a hip fracture, 50% spend time in a nursing home while recovering. Approximately 50% of previously independent individuals become partially dependent, and one third become completely dependent.[76] Only one third of patients return to their prefracture level of function.[77]
Secondary complications of hip fractures include nosocomial infections and pulmonary thromboembolism.
Additional fractures
Patients who have sustained one osteoporotic fracture are at increased risk for developing additional osteoporotic fractures.[59] For example, the presence of at least one vertebral fracture results in a 5-fold increased risk of developing another vertebral fracture. One in 5 postmenopausal women with a new vertebral fracture incurs another vertebral fracture within one year.[78]
Patients with previous hip fracture have a two-fold[79] to 10-fold increased risk of sustaining a second hip fracture. In addition, patients with ankle, knee, olecranon, and lumbar spine fractures have a 1.5-, 3.5-, 4.1-, and 4.8-fold increased risk of subsequent hip fracture, respectively. Site of prior fracture impacts on future risk of osteoporotic fractures independent of BMD such that in postmenopausal women, prior fractures of the spine, humerus, patella, and pelvis are more predictive of future osteoporotic fractures than fractures at other sites.[80]
The World Health Organization fracture-risk algorithm (FRAX) was developed to calculate the 10-year probability of a hip fracture and the 10-year probability of any major osteoporotic fracture (defined as clinical spine, hip, forearm, or humerus fracture) in a given patient. These calculations account for femoral neck bone mineral density (BMD) and other clinical risk factors, as follows[81] :
The National Osteoporosis Foundation (NOF) recommends osteoporosis treatment in patients with low bone mass in whom a US-adapted WHO 10-year probability of a hip fracture is 3% or more or in whom the risk for a major osteoporosis-related fracture is 20% or more.[2] Note that osteoporosis is, by definition, present in those with a fragility fracture, irrespective of their T-score.
Algorithms such as the FRAX algorithm are useful in identifying patients with low bone mass (T-scores in the osteopenic range) who are most likely to benefit from treatment. A study by Leslie et al demonstrated the effects of including a patient's 10-year fracture risk along with DXA results in Manitoba, Canada.[82] The authors found an overall reduction in dispensation of osteoporosis medications as more women were reclassified into lower fracture risk categories.
Although type 2 diabetes mellitus (DM) is associated with a higher BMD, a study by Schwartz et al concluded that for a given T score and age or for a given FRAX score, the risk of fracture is higher in patients with type 2 DM than in those without type 2 DM. The study conclusions were based on data from three prospective observational studies, statistics from self-reported incidence of fractures in 9449 women and 7436 men in the United States.[83]
The FRAX tool has a low sensitivity for predicting fracture risk in perimenopausal and early-menopausal women. In a study by Trémollieres et al, FRAX had 50% sensitivity in the 30% of women in the study at the highest risk.[84] FRAX also does not include risk of falls; 90% of hip fractures[85] and majority of Colles fractures are associated with falls.[86] The Garvan fracture risk tool is not as widely used as the FRAX but is another validated fracture prediction tool that does account for falls, and may be a better tool for use in men.[87]
Vertebral compression fractures often occur with minimal stress, such as coughing, lifting, or bending. The vertebrae of the middle and lower thoracic spine and upper lumbar spine are involved most frequently. In many patients, vertebral fracture can occur slowly and without symptoms.
Hip fractures are the most devastating and occur most commonly at the femoral neck and intertrochanteric regions (see the image below). Hip fractures are associated with falls. The likelihood of sustaining a hip fracture during a fall is related to the direction of the fall. Fractures are more likely to occur in falls to the side; less subcutaneous tissue is available to dissipate the impact. Secondary complications of hip fractures include nosocomial infections and pulmonary thromboembolism.
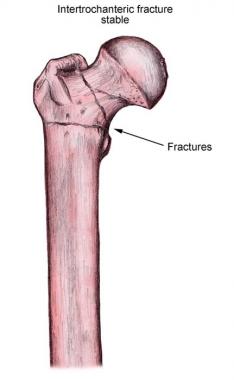 Stable intertrochanteric fracture of the femur.
Stable intertrochanteric fracture of the femur.
Fractures can cause further complications, including chronic pain from vertebral compression fractures and increased morbidity and mortality secondary to vertebral compression fractures and hip fractures. Patients with multiple fractures have significant pain, which leads to functional decline and a poor quality of life (QOL).[88] They are also at risk for the complications associated with immobility, including deep vein thrombosis (DVT) and pressure ulcers. Respiratory compromise can occur in patients with multiple vertebral fractures that result in severe kyphosis.
Patients with osteoporosis develop spinal deformities and a dowager's hump, and they may lose 1-2 inches of height by their seventh decade of life. These patients can lose their self-esteem and are at increased risk for depression.
Patient education is paramount in the treatment of osteoporosis. Many patients are unaware of the serious consequences of osteoporosis, including increased morbidity and mortality, and only become concerned when osteoporosis manifests in the form of fracture; accordingly, it is important to educate them regarding these consequences. Early prevention and treatment are essential in the appropriate management of osteoporosis.
The focus of patient education is on the prevention of osteoporosis. Prevention has 2 components, behavior modification and pharmacologic interventions. Appropriate preventive measures may include adequate calcium and vitamin D intake, exercise, cessation of smoking, and moderation of alcohol consumption.
Patients should be educated about the risk factors for osteoporosis, with a special emphasis on family history and the effects of menopause. Patients also need to be educated about the benefits of calcium and vitamin D supplements, as well as strategies to prevent falls in the elderly (see Primary Care–Relevant Interventions to Prevent Falling in Older Adults: A Systematic Evidence Review for the US Preventive Services Task Force [USPSTF]).
All postmenopausal women older than 65 years should be offered bone densitometry, as well as some younger women and men. These patients should understand the benefits of bone density monitoring. Society at large also should be educated about the benefits of exercise with regard to osteoporosis.
For patient education information, see the Osteoporosis Center, Digestive Disorders Center, and Women's Health Center, as well as Osteoporosis, Anorexia Nervosa, Inflammatory Bowel Disease, and Menopause.
Clinical Presentation
Copyright © www.orthopaedics.win Bone Health All Rights Reserved