

The earliest report of osteochondritis dissecans (OCD) was published in 1888 by Konig, who characterized a loose-body formation associated with articular cartilage and subchondral bone fracture.[1] In 1922, Kappis described this process in the ankle joint.[2]
On the basis of a review of all literature describing transchondral fractures of the talus, Berndt and Harty developed a classification system for radiographic staging of osteochondral lesions of the talus (OLTs).[3] Their classification system has been the foundation for other systems, yet it remains the most widely used system today (see Workup, Staging).
Conservative treatment should be attempted first, whenever possible. Surgical treatment depends on a variety of factors, including patient characteristics and lesions (see Treatment). Surgery is contraindicated when the risks outweigh the perceived benefits.
For patient education resources, see Ankle Arthroscopy, Understanding X-rays, and Magnetic Resonance Imaging (MRI).
NextThe dome of the talus is covered by the trochlear articular surface, which supports the weight of the body. The talar dome is trapezoidal in shape, and its anterior surface is on average 2.5 mm wider than the posterior surface. The medial and lateral articular facets of the talus articulate with the medial and lateral malleoli. The articular surface of these facets is contiguous with the superior articular surface of the talar dome. (See the image below.)
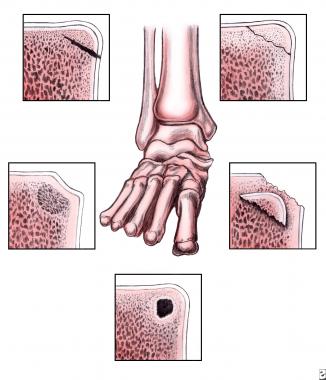 Osteochondral lesions of the talus. Modified staging system by Loomer et al.
Osteochondral lesions of the talus. Modified staging system by Loomer et al.
Approximately 60% of the talar surface is covered by articular cartilage.[4] The talus has no muscular or tendinous attachments. Most of the blood supply of the talus enters through the neck via the sinus tarsi. The dorsalis pedis artery supplies the head and neck of the talus. The artery of the sinus tarsi is formed from branches of the peroneal and dorsalis pedis arteries. The artery of the tarsal canal branches from the posterior tibial artery. The sinus tarsi artery and the tarsal canal artery join to form an anastomotic sling inferior to the talus, from which branches enter the talar neck.
Anterolateral lesions on the talar dome result from inversion and dorsiflexion forces, which cause the anterolateral aspect of the talar dome to impact the fibula. These lesions are usually shallower and more wafer-shaped than medial lesions, possibly because of a more tangential force vector that results in shearing-type forces.[5]
Posttraumatic medial lesions are deeper and cup-shaped. They result from a combination of inversion, plantarflexion, and external rotation forces that cause the posteromedial talar dome to impact the tibial articular surface with a relatively more perpendicular force vector.
A study of the contact pressures on the talus with varying degrees of lateral ligament transections and ankle positions showed that the medial rim of the talus was subjected to high pressures, even without ligamentous transection.[5] Results of another study implicated the difference in cartilage stiffness; the tibial cartilage is 18-37% stiffer than the corresponding sites on the talus.[6]
The results of other studies indicated that the mean cartilage thickness is inversely related to the mean compressive modulus.[7, 8] These findings may lend credence to the clinically observed etiology of OLTs (ie, repetitive overuse syndrome in medial lesions and an acute traumatic event in lateral lesions).
Observations from biomechanical studies suggest that the size of the lesion may alter the contact stresses in the ankle. Statistically significant changes in contact characteristics occur with lesions larger than 7.5 mm × 15 mm; this finding indicates that lesion size may play a role in predicting long-term outcome.[9]
Both Konig and Kappis believed that lesions due to OCD were the result of ischemic necrosis of the underlying subchondral bone that eventually led to separation of the fragment and its overlying articular cartilage.[1, 2]
Inflammation has not been shown to be a significant factor in the etiology of OCD. Therefore, the term OCD may be misleading. In addition, OCD may be mistakenly understood to refer to the common term osteochondral defect. Assenmacher proposed the term osteochondral lesions of the talus (OLTs).[10]
A history of trauma is documented in more than 85% of patients.[11, 12, 13, 14, 15] Pritsch et al reported that a traumatic event preceded 75% of both medial and lateral lesions in 24 patients.[16] Trauma is implicated less often in posteromedial lesions.[17, 18, 19]
Although the etiology of nontraumatic OLTs is unknown, a primary ischemic event may cause this form of the disease. Nontraumatic OLTs can also be familial. Multiple lesions can occur in the same patient, and identical medial talar lesions have occurred in identical twins.[20]
Osteochondral lesions are rare joint disorders. Most often, they affect the knee, followed by the elbow and the talus. Lesions of the talus account for 4% of all osteochondral lesions in the body.[17] However, they have been found in more than 40% of patients after operative treatment of ankle fractures.[21]
Nonoperative treatments of OLTs are associated with published success rates of 45-50%.[22, 23] Operative interventions have repeatedly been reported with significantly better success rates.
In a meta-analysis by Tol et al, the combination of excision, curettage, and drilling has an 85% success rate and better outcomes than does excision and curettage without drilling or excision alone.[22] In a systematic review by Donnenwerth et al, 80.2% good-to-excellent results were identified with arthroscopic debridement and microfracture, regardless of lesion location or lesion size.[24]
Choi et al demonstrated an independent prognostic effect with lesion containment, for which poorer outcomes were identified with uncontained lesions.[25] In this same study, lesion location did not result in an independent prognostic effect. Choi et al identified that a critical defect size related to poor outcomes following arthroscopic marrow stimulation techniques at 150 mm2, correlating to increased risk for a poor outcome.[26]
Autologous osteochondral grafting has likewise had favorable outcomes. Reports on both mosaicplasty and the osteochondral autograft transfer system (OATS) procedure for OLTs have reported success rates around 90% at follow-up of 4 years and 16 months.[27, 28]
The autologous chondrocyte transplantation (ACT) procedure in the knee has been associated with good-to-excellent results at 2-year follow-up.[29] In the ankle, good results have been reported, with arthroscopically confirmed cartilage coverage of the graft site, but whether this newly generated cartilage will hold up to the stresses on the ankle is yet to be seen.[30, 31] A meta-analysis of the available reports identified that the evidence for ACT remains elusive despite a reported 89.9% clinical success rate.[32]
A concept review article revealed grade B (fair evidence from level II or III studies with consistent findings) for bone-marrow stimulation techniques and autologous osteochondral transplantation (OATS or mosaicplasty). Grade C recommendations (conflicting or poor quality [level-IV or V] evidence) were made for osteochondral allograft transplantation and autologous chondrocyte implantation. Biologic adjuncts lacked sufficient evidence to make a recommendation.[33]
Clinical Presentation
Copyright © www.orthopaedics.win Bone Health All Rights Reserved