

Juxtacortical or surface tumors are broadly defined as those arising adjacent to the outer surface of cortical bone. These surface lesions may arise from any of the mesenchymal elements present along the bone surface or from the pluripotent cells found within the periosteum. However, in most cases, it is impossible to determine whether the tumor arose from within the periosteum or from other juxtacortical connective tissues, with secondary involvement of the periosteum. These tumors are composed of a variety of histologic tissue types. The most common types are those producing cartilage or bone.[1, 2, 3, 4]
NextChondroma is a benign tumor composed of mature hyaline cartilage.[5] Typically, it is centrally located, but it may be seen in a juxtacortical position, arising from the periosteum. Periosteal chondromas are rare, constituting 2.2% of benign tumors and 0.5% of all tumors in the Mayo Clinic series.[5] Unlike osteochondromas, which also develop on the surfaces of bones, periosteal chondromas are not related to the physeal plates and most likely develop through subperiosteal cartilage formation.[1, 6, 7, 8]
These lesions occur in patients with a wide range of ages; most occur in persons in the second through fourth decades of life. Unlike osteochondromas, these lesions can continue to grow into adulthood without evidence of malignant transformation. A slight male predominance exists.[5, 9] The most common sites of involvement are the metaphyseal regions of long bones, with the proximal humerus being the most frequently involved bone. They also may involve the bones of the hands and feet.[10]
Patients typically present with a palpable mass and swelling, especially if the lesion is in a superficial location. Patients also may complain of slowly progressive pain due to enlargement of the lesion or from impingement on adjacent soft-tissue structures. On physical examination, the mass is painful, firm, and immobile.[1]
Radiographically, periosteal chondromas demonstrate variable mineralization within the lesion. A shell of new bone formation from the overlying periosteum may also be present (see the first image below). The underlying bone cortex usually is scalloped (saucerization) with various degrees of sclerosis.[11] Cortical sclerosis is well marginated but may demonstrate a buttressing effect at the edges of the lesion. On MRI, the lesion is best seen on T2-weighted images as a well-defined lobular mass (see the second image below). No evidence of tumor extension into the adjacent medullary canal is present (see the third image below).
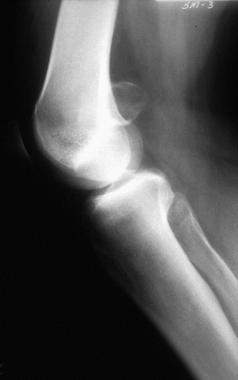 Lateral radiograph of a distal femoral periosteal chondroma; slight mineralization is present within the lesion, with periosteal new bone formation noted at the periphery.
Lateral radiograph of a distal femoral periosteal chondroma; slight mineralization is present within the lesion, with periosteal new bone formation noted at the periphery.
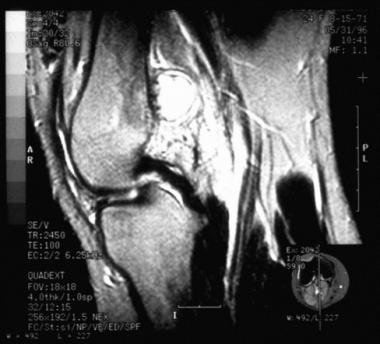 T2-weighted sagittal MRI of a distal femoral periosteal chondroma (same lesion as in the image above). The lobular nature of the lesion is evident, as well as the lack of invasion into the medullary canal.
T2-weighted sagittal MRI of a distal femoral periosteal chondroma (same lesion as in the image above). The lobular nature of the lesion is evident, as well as the lack of invasion into the medullary canal.
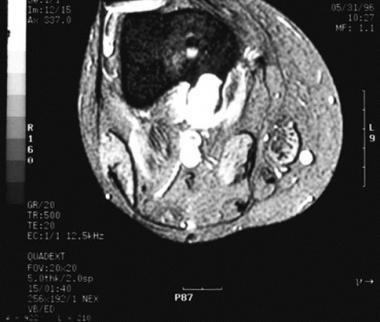 Axial MRI of a periosteal chondroma demonstrating cortical buttressing at the periphery of the lesion, as well as scalloping of the underlying cortex.
Axial MRI of a periosteal chondroma demonstrating cortical buttressing at the periphery of the lesion, as well as scalloping of the underlying cortex.
These lesions tend to be smaller and better marginated than periosteal chondrosarcoma lesions. They may also appear similar to osteochondromas; however, periosteal chondromas do not demonstrate the medullary continuity on plain films and MRIs that is seen with osteochondromas.
Histologically, these tumors are composed of lobules of mature hyaline cartilage, without evidence of permeation into the adjacent bone or soft tissue. Typically, a fibrous capsule overlies the tumor. Areas of the tumor may be hypercellular,[10] typically more cellular than the intramedullary (central) counterpart.[1] Nuclear atypia, binucleation, and pleomorphism also may be noted. Occasional foci of necrosis and myxoid degeneration are present.[12] However, this feature is not as prominent as it is in malignant lesions.
Few somatic mutations have been described for periosteal lesions. Mutations in isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) genes have been described in periosteal chondromas, as well as enchondromas and periosteal and central chondrosarcoma, suggesting similar modes of tumorigenesis. The presence of the mutant enzyme results in accumulation of gamma-2-hydroxyglutarate, an oncometabolite. The presence of this mutation has not been found to differentiate low- versus high-grade peripheral cartilage lesions. However, other periosteal lesions, such as peripheral osteosarcoma, have not been found to demonstrate this mutation.[13, 14]
Malignant degeneration into peripheral chondrosarcoma is extremely rare. Asymptomatic stable lesions can be monitored using serial clinical examinations and plain radiographs.[12, 15] Treatment of the enlarging or symptomatic periosteal chondroma consists of removal of the lesion with a rim of normal bone to decrease the chance of local recurrence. Simple curettage is contraindicated due to the high rate of local recurrence.[10]
Rarely, malignant cartilage may arise within the periosteum.[1] Periosteal chondrosarcoma is more rare than its benign counterpart, periosteal chondroma, with an incidence of 0.15% of all tumors in the Mayo Clinic series. It is differentiated from peripheral chondrosarcoma, which typically arises within the cartilaginous cap of a preexisting osteochondroma.[16] Most patients are in the second to fourth decades of life.[16] They typically present with a slowly enlarging mass and, occasionally, with pain and swelling.[10, 17] These lesions occur most commonly at the distal femur or proximal humerus.[8, 18, 19, 20]
Periosteal chondrosarcoma can be difficult to differentiate from periosteal chondroma. Patients with the former lesions tend to be older, with an average age of 35 years.[1] Further, periosteal chondrosarcomas tend to be larger (see the first image below) and demonstrate invasion into the underlying cortex. However, cortical scalloping and buttressing still may be seen radiographically. On MRI, they are seen as multilobulated lesions, confined to the juxtacortical area, with high signal intensity on T2-weighted images (see the second image below). Typically, no medullary involvement is present.
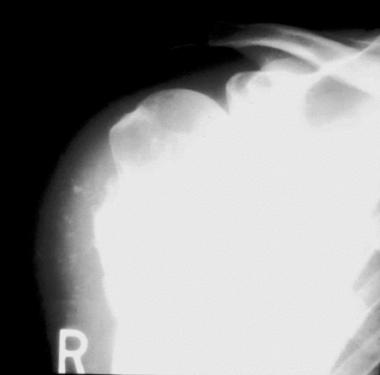 Anteroposterior radiograph of a periosteal chondrosarcoma. Dense mineralization is noted at the base of the lesion. Less well-defined mineralization is noted in the adjacent soft tissues. The lesion is larger than periosteal chondroma lesions.
Anteroposterior radiograph of a periosteal chondrosarcoma. Dense mineralization is noted at the base of the lesion. Less well-defined mineralization is noted in the adjacent soft tissues. The lesion is larger than periosteal chondroma lesions.
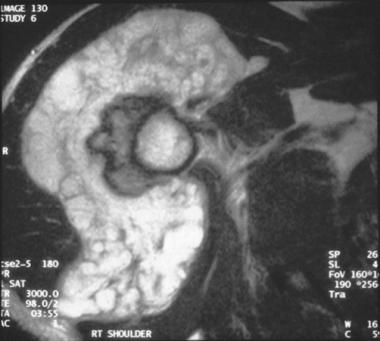 Axial T2-weighted MRI of a periosteal chondrosarcoma demonstrating the lobular nature of the tumor. Erosion into the underlying cortex is present, but the medullary canal remains uninvolved.
Axial T2-weighted MRI of a periosteal chondrosarcoma demonstrating the lobular nature of the tumor. Erosion into the underlying cortex is present, but the medullary canal remains uninvolved.
Histologically, these lesions usually are low-to-intermediate grade. Higher-grade areas occasionally may be seen. levels of matrix metalloproteinases 1, 2, and 9 have been noted in central and periosteal chondrosarcomas, with higher levels of expression noted along with higher grade. Periosteal chondrosarcomas have been found to express these enzymes less consistently than central or dedifferentiated chondrosarcoma, potentially reflecting their typically lower grade[21] Any bone seen within the lesion represents osseous metaplasia or enchondral ossification rather than direct production of bone by the tumor.[16] If the tumor were directly producing bone, the diagnosis would be peripheral chondroblastic or periosteal tumor or osteosarcoma (see Surface Osteosarcoma).
Due to differences in aggressiveness and long-term prognosis, this distinction impacts initial treatment. As noted above, mutations in IDH1 and IDH2 have been noted in both central and peripheral benign and malignant cartilage lesions, including periosteal chondrosarcomas.[22]
Treatment consists of surgical removal with tumor-negative margins. With adequately resected margins, local recurrence is unusual. Metastases also are unusual, and if they occur, they occur late in the disease process.[10]
Like chondrosarcoma, osteosarcoma may be found on the surface of bone. Approximately 4% of osteosarcomas are located in a surface or juxtacortical location.[23] Three subclassifications exist for these surface tumors based on their clinical, radiographic, and histologic presentation. The 3 tumor types are parosteal, periosteal, and high-grade surface osteosarcomas.
Franchi et al, using immunohistochemistry and electron microscopy, found no significant differences among these tumors or between these surface variants and conventional osteosarcoma.[24] However, Chen et al,[25] while investigating the presence of insulinlike growth factor II (IGF2) and mRNA-binding protein 3 (IMP3) in conventional intramedullary, parosteal, and periosteal osteosarcoma, found that the first demonstrated primarily cytoplasmic staining, while the last 2 demonstrated nuclear staining. The authors stated that these different expressions might reflect different roles for IGF2 and IMP3 in tumor angiogenesis, with those tumors expressing more cytoplasmic staining, demonstrating greater microvessel density.
Parosteal osteosarcoma is the most common of the 3 subclassifications,[1] comprising 65% of juxtacortical osteosarcomas,[26] 3-6% of all osteosarcomas, and 2% of primary osseous neoplasms.[10] These lesions are almost exclusively seen around the knee, typically along the posterior surface of the distal femur. Lesions occasionally may be seen at the proximal humerus. Parosteal osteosarcoma occurs in older persons than does conventional intramedullary osteosarcoma. Ninety-five percent of cases occur in persons aged 15-40 years,[10] with peak incidence in persons in the second and third decades of life in the Memorial-Sloan-Kettering series[1] and in persons in the third and fourth decades of life in the Mayo Clinic series.[5] Further, unlike conventional osteosarcoma, there is a slight female predilection (female-to-male ratio of 3:2) noted for the parosteal variant.[27, 28]
Patients with parosteal osteosarcoma typically present with a slowly growing painless mass. Approximately 50% of patients present with a history of symptoms of at least 1 year.[1, 29] Pain and swelling occur late. Because the tumor is adjacent to a joint, patients may demonstrate decreased range of motion of that joint.[10]
Radiographically, parosteal osteosarcoma appears as a radiodense mass on the surface of the bone. It usually is broad-based and may encircle the bone. Periosteal reaction, seen frequently in conventional intramedullary osteosarcoma, is not seen in the parosteal variant. If this is noted, it may represent a conventional osteosarcoma with extraosseous extension. Areas of lucency on plain films suggest the possibility of dedifferentiation, or more aggressive areas, in a portion of the tumor. On CT scan, the areas where the tumor encircles the bone may be seen as a clear space (string sign or cleavage plane) between the tumor and the bone cortex; this area reflects unmineralized, thickened periosteum. CT scan also can delineate areas of cortical erosion seen in most of the patients.[3]
The radiographic differential diagnosis includes osteochondroma and Nora lesion. Although parosteal osteosarcoma is most commonly noted at the distal femur, most series describing patients treated with less than wide margins include only few lesions in this location, indicating the difficulty of making the diagnosis on radiographs when the lesion involves a different bone.[26]
MRI should be performed as part of the initial workup, as it can identify the soft-tissue and, possibly, the intramedullary extent of the tumor. Intramedullary extent appears to have no correlation to outcome, as long as negative surgical margins are obtained. Jelinek et al demonstrated that the intramedullary extent usually is composed of low-grade tumor.[23] Sheth et al found no statistically significant difference in the presence of intramedullary involvement of classic or dedifferentiated parosteal osteosarcoma,[30] although the correlation between the presence of intramedullary involvement and dedifferentiation has not been clearly delineated.[31] MRI aids in preoperative planning to ensure adequate margins.
MRI also helps to identify more aggressive areas within an apparent parosteal osteosarcoma in that a tumor that is entirely low grade demonstrates low signal intensity on T1- and low-to-intermediate signal intensity on T2-weighted images. Tumors with areas of dedifferentiation have areas of high signal intensity on T2-weighted images.[23]
On gross examination, the tumor is densely mineralized. Regional cortical or medullary canal involvement can be demonstrated in approximately 50% of cases.[1] Medullary involvement typically is gross; only a few tumors have histologic involvement only.[32] Microscopically, the tumor is composed of a low-grade spindle cell neoplasm with minimal cytologic atypia.[10] Bone formation occurs within this fibrous stroma. Areas of higher-grade spindle-cell sarcoma with decreased mineralization may be seen, frequently corresponding to radiolucencies on preoperative radiographs. These lesions may also contain cartilage, increasing the difficulty in some cases in differentiating them from osteochondromas.
Typical low grade parosteal osteosarcoma does not express the higher MIB-1 indices or multiple chromosomal aberrations typically seen with higher-grade lesions. To help distinguish low-grade, including parosteal, osteosarcoma from radiographically similar benign lesions, Yoshida et al[33] used immunohistochemistry to assess for the protein products of the oncogenes murine double-minute type 2 (MDM2) and cyclin-dependent kinase 4 (CDK4). They found that 57% of the tumors in their series were positive for MDM2 and 100% for CKD4, while only 1 in 40 benign lesions where positive for either, resulting in 100% sensitivity and 97.5% specificity for the presence of a low grade osteosarcoma.
Dujardin et al,[34] in a similar study, found no immunopositivity among benign lesions or high-grade osteosarcomas and consistent positivity among low-grade osteosarcoma, including parosteal osteosarcoma. In addition, this group found amplification of MDM2 and CDK4 genes among low-grade osteosarcomas, while no amplification was noted among benign or high-grade lesions.
The possibility of higher-grade areas within dedifferentiated parosteal osteosarcoma lesions emphasizes the need for adequate sampling of the tumor at biopsy. This constitutes the dedifferentiated variant of parosteal osteosarcoma. Due to its low-grade nature, as seen histologically, parosteal osteosarcoma has the best prognosis of any of the variants of surface osteosarcoma, with survival rates of 80% noted at 20 years postoperatively in the Memorial-Sloan-Kettering series[1] and 90% at 5 years postoperatively in the Mayo Clinic series[32] using surgery alone.
Treatment of these tumors consists of wide resection and limb salvage when possible. Although low grade, these tumors may recur locally with less than a wide resection margin, and dedifferentiation has been noted in cases with multiple local recurrences.[1]
Okada et al found a statistically significant increase in local recurrence following intralesional or marginal resection versus wide resection (41% vs 0% at 5 years postoperatively).[32] Song et al,[26] in a review of patients referred after intralesional resections of parosteal osteosarcomas, noted a 45% local recurrence rate after repeat resection; the recurrence rate was lower, however, if patients underwent repeat resection prior to the development of gross recurrence. However, wide margins may be difficult to achieve for distal femur lesions due to the proximity of the tumor to the popliteal vessels. Okada et al found that of those parosteal osteosarcomas evaluated with cross-sectional imaging, the neurovascular bundle was invaded in 22% of cases and displaced in 62% of cases. However, this does not necessarily preclude wide resection and limb salvage.
Temple et al believed that accepting close but negative margins over limited regions of the tumor resulted in an acceptable local control rate and less morbidity than if wider margins were sought with segmental resections of the neurovascular bundle.[3]
Metastases in classic parosteal osteosarcoma are rare. In the Mayo Clinic series,[5] no classic parosteal osteosarcomas produced metastatic disease. Likewise, the only occurrence of metastatic disease reported by Kavanagh et al was in a patient with evidence of dedifferentiation at the time of local recurrence.[29] Okada et al found a rate of metastasis of 47% at 5 years postoperatively in those with a dedifferentiated component and 0% in those with classic parosteal osteosarcoma.[32] Funovics et al[35] noted that risk of metastatic disease was related to the presence of dedifferentiation in the tumor, not the presence of medullary involvement. Therefore, systemic treatment is not indicated in cases of classic, low grade parosteal osteosarcoma.
Parosteal osteosarcoma that contains higher-grade dedifferentiated areas has a worse prognosis with a higher risk for systemic metastases. This has been estimated to be present, either in a primary or recurrent tumor, in 22-64% of parosteal osteosarcomas. Treatment of this tumor should include systemic chemotherapy and wide resection. Sheth et al found that in 40% of patients treated neoadjuvantly, the high-grade component of the tumor demonstrated a good response. These patients had an improved survival rate.[30]
Periosteal osteosarcoma represents the second most common type of surface osteosarcoma,[5] accounting for 26 of the 1649 osteosarcomas in the Mayo Clinic series. It occurs in a similar patient population (in persons in the second and third decades of life) as that seen with conventional osteosarcoma.[1] However, a female predilection has been noted.[5] This tumor differs in location from the parosteal variant both in terms of location and radiographic and histologic parameters.[36, 37]
Periosteal osteosarcoma most frequently is seen on the surface of the proximal tibia, although it also has been reported frequently in the tibial and femoral diaphysis.[5] Unlike with the parosteal variant, the posterior aspect of the distal femur rarely is involved (Raymond, 1991). Patients present with a painful enlarged mass. The clinical course progresses more rapidly than that seen with surface cartilage lesions or parosteal osteosarcoma.
On plain radiographs, the lesion demonstrates heterogeneous ossification (see the first image below). Radiolucency may be seen with a greater frequency in periosteal tumors than in parosteal osteosarcoma and can mimic the appearance of myositis ossificans. A history of trauma may point toward the latter lesion, but periosteal osteosarcoma cannot be excluded. An accompanying periosteal reaction may be present in the form of a Codman triangle (see the second image below).[38] Ill-defined erosion or thickening of the underlying cortical bone may be noted (see the third image below).
However, the incidence of intramedullary extent varies among reports, with rare intramedullary involvement noted in some studies, while Cesari et al[39] noted involvement in 70% of patients. Unlike what has been reported for parosteal osteosarcoma, intramedullary involvement may impact the prognosis of periosteal osteosarcoma. In the Rizzoli series,[39] metastases were noted only in patients demonstrating intramedullary involvement; this may indicate a subset of patients with more aggressive tumors.
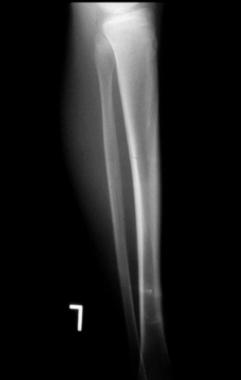 Lateral radiograph of the tibia demonstrating a periosteal osteosarcoma. The lesion shows only vague mineralization in the anterior soft tissues.
Lateral radiograph of the tibia demonstrating a periosteal osteosarcoma. The lesion shows only vague mineralization in the anterior soft tissues.
 Gross specimen of a periosteal osteosarcoma. Lobulated tumor is evident within the surrounding periosteal bone response (Codman triangle).
Gross specimen of a periosteal osteosarcoma. Lobulated tumor is evident within the surrounding periosteal bone response (Codman triangle).
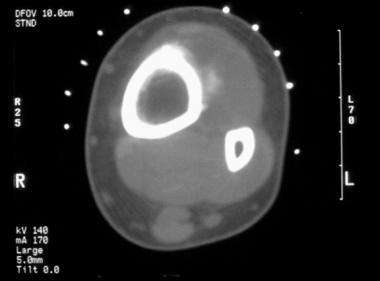 CT scan of the periosteal osteosarcoma seen in the image before the previous image. The soft tissue mineralization is better delineated. However, the mineral within the tumor is not well organized. Underlying cortical erosion is present, without evidence of medullary involvement.
CT scan of the periosteal osteosarcoma seen in the image before the previous image. The soft tissue mineralization is better delineated. However, the mineral within the tumor is not well organized. Underlying cortical erosion is present, without evidence of medullary involvement.
Microscopically, the tumor is composed of moderately differentiated chondroblastic osteosarcoma. The spindle-cell component usually is of intermediate grade, with osteoid production noted within these areas. A pronounced degree of cartilage may be present within this tumor, contributing to the lobulated appearance seen on gross sections. However, the chondrocytes also may demonstrate some atypia.[10]
Treatment consists of wide surgical resection. The Mayo Clinic series reported an 80% survival rate with surgical treatment. An 84% 10-year survival rate was noted in a report from the Rizzoli Orthopaedic Institute[39] ; patients in this series were treated with surgical resection, with some patients also receiving chemotherapy. A metastatic rate of 15% has been reported with local therapy alone.[16] Due to this and to the higher-grade nature of some of the tumors, systemic chemotherapy may be used. However, the role of routine systemic treatment remains unclear.[38] In the Rizzoli series,[39] no differences were noted in 10-year event-free or overall survival between the group of patients treated with surgical resection and chemotherapy compared with those treated with surgery alone. The overall survival rate is approximately 80% in the Mayo Clinic series.[40]
High-grade surface osteosarcoma is the least common of the surface osteosarcomas, accounting for 12 of 1649 osteosarcomas in the Mayo Clinic series.[5] Like conventional intramedullary osteosarcoma, the high-grade surface variant typically is seen in persons in the second decade of life. In the Mayo Clinic series, 70% of patients were in the second or third decades of life.[40] Also like conventional osteosarcoma, a male predominance exists (male-to-female ratio of 3-4:1).[5, 40, 31, 41]
Patients typically present with an enlarging painful mass and swelling. Unlike the other surface osteosarcomas, duration of symptoms prior to diagnosis of this variant is short; most patients present within 1-6 months.[40] The tumor most commonly is located in the mid or distal femur or in the tibial diaphysis.
On plain radiographs, lesions demonstrate a broad attachment to the cortex. However, they do not encircle the cortex as seen in parosteal osteosarcoma. Poorly marginated involvement of the underlying cortex may be evident, either in the form of cortical erosion or thickening.[40] The lesion has no specific radiographic features and may be radiolucent or may demonstrate a radiodense sunburst appearance on the surface of the bone.[42] Medullary involvement may be difficult to detect but is best assessed preoperatively on MRI, especially on T2-weighted images. Thirty-three percent of the tumors in the Mayo Clinic series that were evaluated with cross-sectional studies demonstrated medullary involvement. Extensive medullary involvement suggests the diagnosis of conventional intramedullary osteosarcoma with extraosseous extent. As with all 3 types of surface osteosarcoma, the prognostic significance of medullary involvement is unclear.
High-grade surface osteosarcoma is histologically identical to the conventional intramedullary form. Sheets of pleomorphic spindle cells produce the osteoid matrix. High-grade surface osteosarcoma can be distinguished from dedifferentiated parosteal osteosarcoma by a lack of adjacent low-grade areas.
The prognosis for high-grade surface osteosarcoma is the worst of surface osteosarcomas and is similar to that of the conventional type. Therefore, these tumors should be approached aggressively, with systemic preoperative and postoperative chemotherapy and wide surgical resection. The long-term survival rate is less than 33% with adequate surgical resection alone. Local recurrence is significantly associated with a less than wide margin. The overall prognosis is affected by the initial grade of the tumor, response to neoadjuvant chemotherapy, and the presence of local recurrence.
Copyright © www.orthopaedics.win Bone Health All Rights Reserved