

Osteoid osteoma is a benign osteoblastic tumor that Bergstrand first described in 1930.[1] Jaffe described it in 1935 and was the first to recognize it as a unique entity.[2] Osteoid osteomas are usually smaller than 1.5-2 cm and characterized by an osteoid-rich nidus in a highly loose, vascular connective tissue. The nidus is well demarcated and may contain a variable amount of calcification. Surrounding the nidus is a zone of sclerotic but otherwise normal bone.[3, 4, 5, 6]
Osteoid osteoma can occur anywhere. It can involve a single bone or several bones. Osteoid osteoma is reported to occur in the cortex of the shafts of long bones in 80-90% of cases. It is also reported in the epiphyseal and metaphyseal regions of both small and large bones of the axial and appendicular skeletons, especially the femur, tibia, and humerus.
The lower extremities are the most common sites of osteoid osteomas. The femur, particularly the intertrochanteric or intracapsular regions of the hip, is affected in two thirds of cases.[7, 8] The diaphyseal part of the tibia and the humerus are other common sites. Barei et al reported that in 50-60% of cases, osteoid osteoma occurs in the femur and tibia.[9, 10]
Approximately 7-20% of osteoid osteomas involve the spine. Involvement here most commonly manifests as painful scoliosis, but painless conditions can also occur. Pettine et al noted that 50% of lesions occur in the cervical spine, and up to 78% of osteoid osteomas in the lumbar spine are associated with scoliosis.[11, 12, 13, 14]
The tumor has a predilection for the posterior elements, most commonly affecting the cancellous lamina, spinous process, and pedicle but sparing the vertebral bodies. Wells et al observed this predilection in 75% of cases, with 33% involving the lamina, 20% involving the articular facets, and 15% involving the pedicles.[15] About 59% of osteoid osteomas affect the lumbar spine. Rates in other areas are 27% in the cervical spine, 12% in the thoracic spine, and 2% in the sacrum.
Other areas that may be involved include the hand, talus, foot, and joints.[16, 17, 18] Osteoid osteomas of the hand and wrist are rare, most commonly involving the phalanges, and often result in atypical clinical and radiologic characteristics. The findings are similar to those of tumors involving the foot and ankle. Intra-articular osteoid osteoma occurs in 10% of cases and can involve the hip, elbow, and ankle.
Osteoid osteoma is a relatively common bone tumor, accounting for approximately one eighth to one tenth of all symptomatic benign bone tumors and 5% of all primary bone tumors.
Osteoid osteoma is generally a condition of the young, but it can affect a wide range of individuals aged 8 months to 70 years. The literature reports that people aged 10-30 years are most susceptible. About 90% of cases occur in patients younger than 25 years. In fact, Barei et al noted that 70% of osteoid osteomas occurred in patients younger than 20 years.[9] The tumor is less common in patients older than 30 years, accounting for 13% of cases. About 3% of cases occur in children younger than 5 years.[19, 20]
Men are affected more frequently than women. The male-to-female ratio is 2-3:1.
No racial or ethnic predilection is noted for osteoid osteoma.
Clinical picture
Patients with osteoid osteoma can present with an atypical history and lesions in unusual locations. Because of this presentation, osteoid osteomas can be confused with osteomyelitis, especially Brodie abscesses, eosinophilic granulomas, and other benign cysts.
Radiographic appearance
Radiographic findings of osteoid osteoma may mimic those of stress fractures, intracortical abscesses, sclerosing osteomyelitis of Garré, or avascular necrosis. It should be differentiated from osteochondritis dissecans and inflammatory arthritis. On rare occasions, it can resemble osteosarcoma.
Features on bone scanning
Histologically, osteoid osteoma is almost identical to osteoblastoma, osteosarcoma, and enostosis. However, benign osteoblastoma has a uniform pattern of thick, closely packed trabeculae with increased cellularity and vascularity. Osteosarcoma and parosteal osteosarcoma are more cellular than osteoid osteoma, and they are anaplastic with elaborate malignant osteoid. Enostosis, on the contrary, represents the opposite extreme, with islands of densely packed, thickened bone trabeculae with normal stroma.[21]
Histologic appearance
Osteoid osteoma can appear as spondylolysis on bone scans; both can appear as abnormal activity in the spine. Wells et al noted that in cases of spondylolysis, immediate postinjection images showed minimal or no abnormal activity but that abnormal activity was observed when imaging was delayed after the injection.[15] Early uptake of tracer is detectable in all cases of osteoid osteoma. Delayed images reveal unilateral or bilateral abnormalities in cases of spondylolysis but demonstrate intense tracer accumulation in cases of osteoid osteoma.[22]
Natural history
The natural history of osteoid osteoma is controversial. The literature suggests a history of resolving pain and healing of the lesions, but no histologic confirmation of the diagnosis has been reported. The course of this disease is unpredictable and protracted, with intervals of resolution of pain that sometimes last 6-15 years.
Atar et al (1992) described two stages of the disease.[23] The first is an acutely painful stage that lasts 18-36 months, during which patients require steady use of analgesics. The second is the recovery stage, which includes healing of the nidus and which usually takes 3-7 years. Barei et al noted that healing involves ossification of the untreated nidus, which cannot be readily distinguished from surrounding bone and which resembles a localized zone of cortical hypertrophy.[9]
NextSymptoms of osteoid osteoma can last from weeks to years before diagnosis and eventual surgery.
Pain is the principal symptom of both initial and recurrent disease. It is described as a continuous, deep, aching, and intense pain with varying quality and severity. It is typically localized to the site of the lesion. Pain is usually worse at night (in 95% of patients) and diminishes by morning. The pain may awaken the patient (29% of cases). Some patients may have an exertional component, especially those with intracapsular lesions with synovitis and restricted range of motion. The pain may also affect the patient's gait.
The pain usually occurs before the lesions are visible on radiographs. The pain is responsive to oral salicylates, often with dramatic beneficial results, but the response is not universal. In approximately 50-75% of patients, it responds to oral salicylates. Pain symptoms can be exacerbated with the use of ethanol.
Osteoid osteoma should be considered in any young patient with pain in the back or neck, painful scoliosis, or radicular or referred-type pain into the lower limb or shoulder. Its symptoms can simulate those of a herniated disk, or the lesion may produce radicularlike symptoms in the shoulders and arms. Unexplained, rigid, or painful scoliosis, especially if the pain is referred to the concavity of the curve, has been associated with osteoid osteoma. Local pain is most commonly noted in the area of the tumor.
Swelling can also occur in osteoid osteoma and is sometimes the only presenting symptom. This is usually observed in patients with diaphyseal lesions.
Osteoid osteoma involving the hand can manifest as monoarticular arthritis,[24] macrodactyly, clubbing, and painless swelling with absence of reactive bone or bony lysis.[25]
Physical findings can vary in patients with osteoid osteoma. Tenderness is present in 62% of patients and usually occurs with subperiosteal lesions; it is relatively uncommon with medullary lesions. Local warmth and erythema are possible but unusual.
Intracapsular lesions are rare. Epiphyseal lesions mimic intra-articular derangement and can delay the true diagnosis. These lesions may be associated with proliferative synovitis due to prostaglandin secretion that decreases range of motion. Joint effusion can be present, mimicking inflammatory arthritis. Soft-tissue swelling, contractures, and a soft-tissue mass can also be noted. Radiographs may reveal the lack of an intense perifocal sclerotic margin and perinidal bone marrow edema.
Neurologic findings are variable. Janin et al and MacLellan et al noted a 25% incidence of neurologic abnormalities.[26, 27] Reports in the literature describe fewer neurologic abnormalities, such as monoparesis and paraparesis, with osteoid osteoma than with osteoblastoma because osteoid osteomas are smaller than osteoblastomas.
Patients with osteoid osteoma usually have normal blood and chemistry findings.
Osteoid osteoma has been linked to prostaglandins, which may explain the inflammatory features of the lesion. Some authors have postulated that prostaglandins may have a fundamental role in the development of osteoid osteoma.
Several authors have noted extremely high levels of prostaglandin metabolites, especially prostaglandins E2, I2, and F1 α, which have been seen at levels 100-1000 times normal levels, especially in the nidus.
Two pathways have been postulated for pain generation in osteoid osteoma secondary to the effects of prostaglandin. One pathway involves vasodilatory and permeability effects related to an increase in both the size of blood vessels and flow in blood vessels in the bony lesion that increases pain and pressure. In the second pathway, the effects of the bradykinin system potentiate pain akin to that due to injured soft tissue because of increased capillary permeability.
Delays of 11.8-36 months have been reported in the diagnosis and treatment of osteoid osteoma. Pettine et al noted that bone scanning dramatically decreased the mean interval from the appearance of symptoms to diagnosis from 35 months to 12 months.[11]
Radiography
Osteoid osteoma elicits a profound osteoblastic response in surrounding medullary and cortical bone and shows the characteristic picture of sclerosis around a lucent nidus. It appears as cortical thickening and diffuse medullary sclerosis on radiographs (see the image below). Radiography usually reveals a radiolucent area of about 1 cm in diameter, called the nidus, with a center that is sometimes calcified, resulting in a radiopaque point called the bell. The nidus is surrounded by a rim or halo of radiodense cortical hypertrophy or hyperostosis.
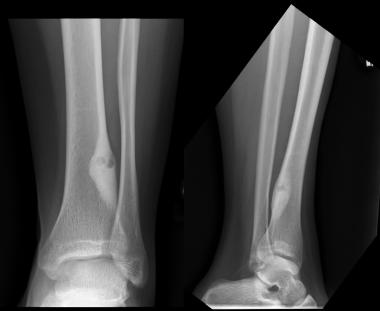 Anteroposterior (AP) and lateral radiographs of osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
Anteroposterior (AP) and lateral radiographs of osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
Cortical lesions may appear with sclerosis that may obscure the lucent nidus.
Intracapsular lesions usually manifest as proliferative synovitis with joint effusion and soft-tissue swelling and no sclerosis. Atar et al noted that lesions in the femoral neck caused widening and foreshortening of the femoral neck, with a reduction in the height of the capital femoral epiphysis and with osteoporosis of the proximal femur.[23]
Subperiosteal lesions may not be apparent on plain radiographs.
Epiphyseal and metaphyseal lesions may show only minimal sclerotic changes around the nidus.
Variation in radiographic appearance is possible. Lesions can appear as only a radiolucent zone, only a radiopaque area, or as a central sclerosis with surrounding radiopacity.
Regarding the diagnostic value of radiography, some authors have reported that the combination of clinical and radiographic features confirms the diagnosis. However, Georgoulis et al noted that plain radiographs alone have low diagnostic value in detecting the lesion.[28]
Osteoid osteoma may be present months to years before radiographic confirmation. Radiographs may be normal during the first months after the onset of complaint. Therefore, repeat radiographs should be obtained from time to time to document osseous manifestations.
Radionuclide scanning
Radionuclide scans are reliable tools when radiographic findings are not diagnostic. Wells et al urged that bone scans be performed when radiographic findings are normal or inconclusive, especially in pediatric patients with back pain. Radionuclide concentrates principally in the nidus of osteoid osteoma and minimally in perifocal bone.[15]
Osteoid osteoma is associated with a nonspecific but intense, well-defined uptake of activity on bone scans. This focus of intense uptake is correlated with the nidus. Meire et al reported the presence of a nonspecific hot spot long before radiographs depict an alternation of bone texture that represents an osteoblastic lesion.[29]
Technetium bone scanning aids in establishing the diagnosis of osteoid osteoma. Rinsky et al reported that technetium-99m scintigraphy is sensitive for osteoid osteoma in its early stages.[30] In fact, the diagnostic delay is reduced from 35 months to 12 months with the use of bone scans. Swee et al reported that 25% of patients with osteoid osteoma presented with negative radiographic findings but positive bone scans.[31] They recommended that bone scanning be done in suspected cases when radiographic findings are normal.
Radionuclide scanning also has the advantage of aiding the surgeon and the pathologist in confirming resection of the tumor and locating the lesion for histologic examination. Specimen autoradiography and scintigraphy are accurate in localizing the nidus, thus facilitating histologic examination.[32, 33, 34]
Bone scintigraphy also is helpful in ruling out multicentric processes.[35, 36]
To date, no negative bone-scan findings have been reported in patients with osteoid osteoma. Bone scanning is currently the most accurate means of localizing the tumor. Wells et al noted that the sensitivity of skeletal scintigraphy for osteoid osteoma is 100%.[15]
Osteoid osteoma can sometimes be confused with spondylolysis, especially in the lower lumbar spine, as both result in a hot spot on bone scans. Wells et al observed two basic differences between the diseases, as follows.[15]
Images obtained immediately after injection showed minimal or no abnormal activity in spondylolysis.[15] By comparison, images of osteoid osteoma demonstrated easily detectable uptake of the tracer in all cases. However, abnormal activity was noted with spondylolysis when a delay occurred between injection and imaging. On delayed images, both spondylolysis and osteoid osteoma showed hot spots, but spondylolysis resulted in unilateral or bilateral abnormalities, whereas osteoid osteoma led to intense tracer accumulation.
Arteriography
Arteriography can be used when other examinations fail to give sufficient information. Meire et al described three phases of osteoid osteoma on the basis of arteriographic findings[29] :
Computed tomography
Computed tomography (CT) is helpful in precisely delineating the nidus. It is recommended when the nidus is not visible on conventional radiography, when residual or recurrent tumor is present, or when the tumor is located in a critical area (eg, spine or femoral neck). CT increases specificity for calcified lesions and allows visualization of the nidus. It is helpful in precisely defining the location of the tumor and the extent of osseous involvement, especially in areas deep in complex joints (eg, the hip).[37, 38] (See the images below.)
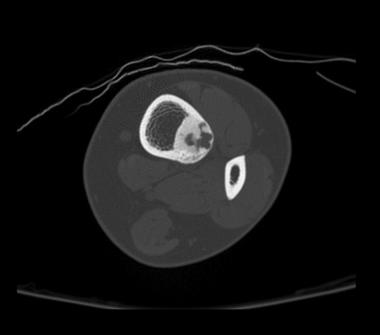 Axial CT scan shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
Axial CT scan shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
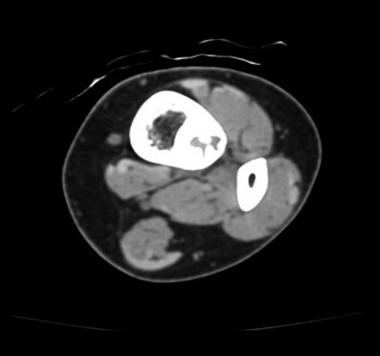 Reconstructed axial CT scan shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
Reconstructed axial CT scan shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
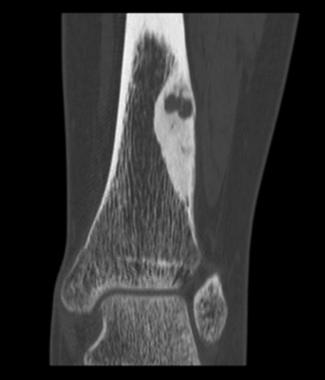 Coronal CT scan shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
Coronal CT scan shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
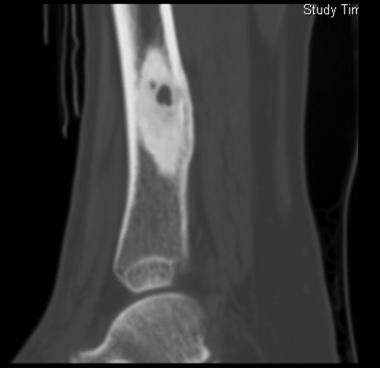 Sagittal CT scan shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
Sagittal CT scan shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
CT is more accurate than magnetic resonance imaging (MRI). Sans et al reported that CT helped in confirming the diagnosis of osteoid osteoma in 74% of cases.[39] Szendroi et al reported accuracies of about 66% in the diagnosis of intra-articular lesions and 90% in extra-articular lesions.[40] At present, CT is the primary investigational tool for the definitive diagnosis of osteoid osteoma.[41]
On CT scans, osteoid osteoma appears as a circumscribed annular lesion with a double-attenuating sign. When CT is performed with intravenous contrast material, scans of osteoid osteoma typically show a rapid, early arterial phase of enhancement and then a slow exit of the contrast material from the nidus, consistent with delayed flow in the venous phase.
Magnetic resonance imaging
A noncalcified nidus has homogeneous enhancement on contrast-enhanced, fat-suppressed T1-weighted MRI. By comparison, a calcified nidus has ring enhancement, the intensity of which is proportional to the extent of the remaining part of the vascularized nidus.
On T1-weighted MRI, the lesion is an area of decreased signal intensity, sometimes with a bell of highly decreased signal intensity at its center. The degree of bone marrow and soft-tissue enhancement is directly correlated with the size and reactive inflammatory changes of the lesion.[42, 43, 44] (See the images below.)
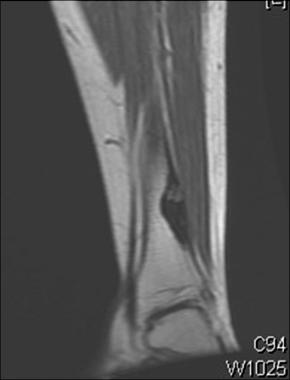 MRI shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
MRI shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
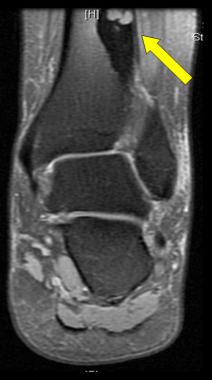 MRI shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
MRI shows osteoid osteoma. Courtesy of Cincinnati Children's Hospital Medical Center, Department of Pediatric Orthopaedics.
MRI has not been useful in the diagnosis of osteoid osteoma. It is reserved for equivocal cases because it can suggest the diagnosis of osteoid osteoma. It is sensitive in detecting bone marrow, peritumoral edema, and soft-tissue abnormalities. Localization of the nidus is often difficult because of an abundance of reactive bone and edema surrounding the lesion that is occasionally misleading.
Assoun et al noted that MRI interpretation resulted in notable errors in diagnosis, most often confusion with malignancies.[45] Guzey et al confirmed this finding. They reported that spinal osteoid osteoma with changes on paravertebral soft tissue can mimic malignant soft-tissue tumors on MRI.[46]
The potential rate of missed diagnosis with MRI is 35%. The tumor is identified on only 65% of axial images. Hence, reliance on MRI alone may lead to clinically significant misdiagnoses. This is particularly true for medullary lesions.[47]
Preoperative localization
Regardless of the method of treatment, its success highly depends on preprocedural localization of the nidus. In fact, most authors agree that exact localization of the lesion is the most important determinant of successful operative removal.
Careful radiographic imaging is necessary to plan surgical treatment. However, radionuclide scanning and CT have been most helpful in localizing the tumor. Radionuclide scanning assists in the localization and diagnosis of osteoid osteoma in its early stages. It is also used in ruling out multicentric processes, which can occur in osteoid osteoma. On the other hand, CT can assist in preoperative localization, and it is useful in precise localization of the nidus in the hip or the spine.
Intraoperative localization
Various methods have been used for intraoperative localization and identification of osteoid osteomas to minimize bone resection and to help ensure complete excision of the tumor.
Klonecke et al reported that intraoperative scanning has evolved because of the excessively wide excision necessary in the past that had to be planned to help guarantee complete removal of the lesion.[48] However, even with the use of frozen sections, adequate excision is not ensured.
Tetracycline fluorescence under UV light requires patients to take tetracycline before surgery (4 mg/kg body weight by mouth four times daily for 1-2 days). The difficulty of intraoperative identification of the nidus often leads to nonexcision of the lesion. Information must therefore be obtained from the excised bone with pathologic examination. If the nidus is not in the specimen, further resection of the bone is required, resulting in unnecessary and excessive removal of bone.
Intraoperative radiography can be helpful in localizing the lesion in reference to a guide pin inserted into the bone. Multiplanar fluoroscopy can help in localizing the nidus if it is in cancellous bone, where sclerotic reactions are minimal. By comparison, cortical lesions contain reactive sclerosis that may obscure the nidus, leading to incomplete removal of the lesion and resulting in recurrence.
Rinsky et al first described intraoperative radioisotope scanning for osteoid osteoma.[30] Advantages include intraoperative localization by radionuclide scintillation probe that is easy and reliable, minimizes bone resection, and does not prolong surgery. It can aid the surgeon and pathologist in confirming resection of the tumor and in localizing the lesion for histologic examination. It is the only technique that aids in verifying complete surgical excision of the lesion (success rate, 94%). Furthermore, it keeps procedural morbidity at its lowest level.
CT is helpful for precise localization of the nidus in the hip or spine. It is also helpful in precisely defining the location of the tumor and the extent of osseous involvement.
Whether the pathophysiology of osteoid osteoma is neoplastic or inflammatory has been a controversial subject. The presence of atypical cellular and trabecular components support the view that osteoid osteoma is a neoplasia; however, the relatively small size of the lesion, its self-limited nature, and the presence of intracellular viral particles (as observed on electron microscopy) may suggest an inflammatory process.
Pain in osteoid osteoma has been typically attributed to the nidus, with its associated hyperostosis and neural elements in the reactive fibrous tissue.
Golding described radially oriented trabeculae of surrounding reactive bone, which implied an increased pressure in the vascular nidus.[49] This arrangement of the bony trabeculae was attributed to the stresses placed on them. This increased pressure due to vasodilatation and edema is thought to directly stimulate intraosseous nerve endings, generating pain.
Schulman et al supported this observation, finding increased amounts of unmyelinated nerve fibers, with greatest abundance next to arterioles. These fibers were believed to be sensitive to changes in vascular pressure.[50]
Prostaglandins have been implicated and linked to osteoid osteoma. Several authors have even suggested that they may have a fundamental role in the development of osteoid osteoma. Support is derived from reports of a 100- to 1000-fold increase in levels of prostaglandins, particularly prostaglandins E2 and I2 (prostacyclin), in the nidus that was reversible on extirpation of the tumor.
These prostaglandins and other mediators of bone formation and inflammation are believed to provide the final common pathway for pain generation. Furthermore, the dramatic response to salicylates or nonsteroidal anti-inflammatory drugs (NSAIDs), which affect prostaglandin synthesis, supports the suggested role of prostaglandins in the pathophysiology of pain.
Healey and Ghelman described two pathways of pain generation due to prostaglandins.[51] The first involves permeability and vasodilatory effects, which increase the size and flow of vessels in the bony lesion, increasing pressure and pain. The second involves its effect in the bradykinin system, which potentiates pain akin to injured soft tissues.
The diagnosis of osteoid osteoma can be confirmed only with pathologic examination.
Osteoid osteoma typically consists of a discrete central nidus, usually smaller than 1 cm with diffuse peripheral sclerosis. The nidus is usually a distinct, well-circumscribed cavity, surrounded by dense reactive bone of varying thickness. It is typically cherry-red in color and can be shelled out of the surrounding reactive bone. The nidus varies in consistency from vascular, soft, friable, gritty, and granular to densely sclerotic.
During surgery, an increased number of fine, punctuate vessels and adherent periosteum overlying the lesion may be observed. Osteoid osteoma is usually cortical and may extend into the periosteal or endosteal surface of the bone. It is rare in the spongiosa.
On microscopic evaluation, the nidus is typically composed of a mass of irregular osteoid tissues that lie in a highly vascular stroma of connective tissue containing osteoblastic cells. It consists of irregular lacelike osteoid and calcified matrix lined by plump osteoblasts and osteoclasts with a well-vascularized but bland stroma.
The microscopic appearance of osteoid osteoma may vary with the degree of lesional maturity. During the initial stage of the disease, proliferation occurs in osteoblasts and vascularized spindle cell stroma with minimal new bone formation. In the intermediate stage, patches of calcified osteoid between the neoplastic stromal cells appear. The mature stage manifests with densely packed atypical bony trabeculae with decreased vascularity and stroma.
The histologic stage is not correlated with the patient's clinical picture.
Pathologic confirmation of the diagnosis of osteoid osteoma by means of percutaneous needle biopsy yields reliable results in only 50% of cases. Use of autoimaging and scintigraphy has increased in the pathologic examination of osteoid osteoma specimens. Autoimaging helps direct attention to the hottest fragment, which corresponds to the nidus. Thus, it decreases false-negative reports, which most commonly occur because of sampling error.
Initial treatment of osteoid osteoma remains nonoperative, with medications consisting of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs). In fact, Barei et al suggested that patients with osteoid osteoma be treated with NSAIDs if they can tolerate them, because many patients may achieve lasting pain relief with NSAIDs.[9]
The response to salicylates is not universal, however; it can vary and is therefore not as reliable a sign as it was previously thought to be. Healey et al noted improved symptoms in 73% of patients, with patients taking only aspirin 650-3250 mg/day to control pain.[51] Kirchner et al also reported that the response to salicylates can vary, from 30% to 75%.[52] Pettine et al described substantially decreased pain with aspirin or NSAIDs, with positive responses in 90% of patients.[11]
Response to other NSAIDs, such as naproxen, has also been reported in the literature. Saville et al noted good responses to therapeutic doses of naproxen after trials with aspirin, indomethacin, ibuprofen, and fenoprofen were tried.[53] They noted resolution of pain after 22 months of treatment, with complete resolution of pain after 33 months. Carpintero-Benitez et al noted a good response of pain to cyclooxygenase-2 (COX-2) inhibitors, as compared with conventional NSAIDs.[54]
Several authors have suggested that because the general mechanism of action of aspirin and NSAIDs is inhibition of prostaglandin synthesis leading to pain relief, any intervention that decreases the concentration of prostaglandins in the osteoma will also decrease the related pain.
If medical management is selected, blood counts and serum chemistries should be periodically monitored in all patients. Sequential radiographs should also be obtained at 3- to 6-month intervals during treatment. Observed radiographic changes that suggest healing of the lesion are ossification of the nidus and increased bone formation around the nidus.
Most patients are unable to continue this regimen of treatment. According to Barei et al, the most common reasons for treatment failure were little tolerance for ongoing pain among young, active patients, who opt for an aggressive surgical alternative; an initial effect of anti-inflammatory medications that diminishes over time; and persistent pain.[9]
Kneisl et al mentioned several contraindications for NSAID use, including sensitivity to the medications, progressive deformity of the limb, and uncertainty about the diagnosis. They added the relative contraindications of patient preference, breakthrough pain with therapeutic doses of medications, and adverse effects (including gastrointestinal toxicity, central nervous system symptoms, and dermatologic manifestations).[55]
Surgical intervention is generally indicated for patients whose pain is unresponsive to medical therapy, patients who cannot tolerate prolonged use of nonsteroidal anti-inflammatory drugs (NSAIDs), and those who are not amenable to activity restrictions.
Lesions in anatomically inaccessible areas, such as the femoral head and neck, result in considerable surgical morbidity, and their removal may cause complications or disability more severe than that associated with the original condition.
Other disadvantages include difficulties in identifying and localizing the nidus at the time of surgery, postoperative restriction of activity that may be required after bone is removed, and an awkward anatomic location of the tumor that may require an extensive surgical approach.
Timing of surgery has not been an issue for osteoid osteoma anywhere in the body except for the spine.
Mehta and Murray reported that patients with scoliosis reach a critical point after which the continuing discharge of painful stimuli results in a structural change of the spine that precludes resolution of the deformity after the tumor is removed.[56] In their study, painful symptoms that were present during the patient's growth spurt were most likely to cause progression of the scoliosis. Furthermore, the patient's age at the onset of symptoms and the duration of symptoms were the most important determinants at this critical point.
Pettine et al observed that 15 months was the critical duration of symptoms for antalgic scoliosis to spontaneously correct after excision.[11] In patients with symptoms lasting less than 15 months, scoliosis is decreased or completely corrected within a short time after excision alone. Age was a less important factor than symptom duration, but patients who were relatively old at onset of symptoms and those who were young at time of surgery were most likely to have spontaneous correction of the curve.
Complete surgical excision of the nidus, even if it is intralesional, is the treatment of choice for patients with osteoid osteoma in whom conservative management fails. It provides immediate relief and is usually curative. Complete surgical excision is the most predictable way to cure osteoid osteoma and should be the goal of surgical intervention. Pettine et al added that the most important determinant for successful surgical removal is the exact localization of the lesion.[11]
The data from one study noted that while conventional excision therapy is effective and reliable, a minimally invasive approach by video-assisted endoscopy, microscope, or percutaneous radiofrequency coagulation results in less tissue trauma and collateral damage and may be an alternative method of treatment.[57]
Campanacci et al suggested two main approaches in open surgical treatment of osteoid osteoma: wide en-bloc resection and unroofing and excision.[58]
En bloc resection
En-bloc resection of the lesion with surrounding bone is recommended in the treatment of osteoid osteoma, but this is not always possible. The purpose is to ensure complete removal of the nidus to minimize the risk of recurrence.
Disadvantages of this procedure include excessive resection of normal bone in the effort to completely excise the lesion. The procedure is contraindicated in patients with lesions in areas difficult to access, such as the acetabulum or femoral head and neck, where it leads to substantial morbidity.
Kruger et al suggested that en bloc excision under imaging intensification is the treatment of choice for epiphyseal lesions.[59]
Unroofing and curettage
The procedure of unroofing and curettage entails unroofing of the nidus by gradually removing the overlying reactive bone, followed by excision with curettes and burrs. It is effective and has a cure rate of 75-100%. Unroofing and curettage is especially helpful in treating lesions in a structurally vital location, such as the femoral neck, wherein the tumor is eradicated without disrupting the underlying central sclerotic bone. This approach helps maintain structural support for the area.
Prophylactic internal fixation
Healey et al reported that prophylactic internal fixation is indicated if substantial cortex is resected and if the remaining bone is weakened.[51]
Spinal fusion
Healy et al reported that spinal fusion should be performed only if instability results from excision of the lesion.[51]
Subperiosteal lesions may be visible as a superficial deformation of a few millimeters in size. Changes on the bone surface in the form of a focal increase in the diameter of the superficial bone vessels, a pink area of millimetric dimensions, or both may reveal the presence of such lesions.
Intracortical lesions are difficult to localize by just scrutinizing the bone surface if it is covered by thick periosteum. However, these lesions cause extensive bone reaction that typically surrounds the nidus of the tumor.
Intra-articular or medullary types cause little or no periosteal reaction; therefore, they are difficult to identify visually.
En-bloc surgical resection of the tumor leads to extended hospital stay; perioperative fractures; the need for bone grafts, internal fixation, or both; periarticular stiffness; and delayed functional recovery.
About 9-28% of osteoid osteomas recur after surgical extirpation, where the rate of local recurrence is inversely proportional to the aggressiveness of the surgery. Healey et al noted that intralesional resection or curettage had the highest recurrence rate and that en bloc resection had the lowest rate.[51] Several authors linked this finding to incomplete removal of the nidus. Recurrence is typically observed within 1 year after excision; hence, the patient should be monitored for a minimum of 1 year.
Proper preoperative and intraoperative localization of the tumor is critical to ensure adequate resection of the tumor and minimize the likelihood of recurrence. The use of these techniques dramatically reduce the recurrence rate.
Failure to relieve pain is associated with a bad prognosis, and it most probably indicates incomplete removal of the tumor. Rosenthal et al noted persistence of symptoms in 30% of cases.[60, 61]
Direct visualization and intralesional excision of the nidus is associated with a primary cure rate of 100%. Successful excision substantially eliminates tumor-related pain within hours to days after surgery. Assenmacher et al described immediate relief of pain in their patients, with a mean symptom-free duration of 6.6 years after surgery.[62]
Radionuclide-guided excision
The use of radionuclides in surgery started in 1949, when attempts were made to facilitate surgery by taking advantage of the property of certain lesions to concentrate radiopharmaceuticals.
For radionuclide-guided excision, patients undergo bone scintigraphy at diagnosis by using technetium-99m-labeled hydroxymethylene diphosphonate (HMDP) and dichloromethylene diphosphonate (DMDP). Three hours before surgery, the radionuclide is given to the patient, followed by control scanning encompassing the hot spot 1 hour after the injection. A radiation detector probe is then used to locate the hot spot by using the signal produced.[32, 63, 64, 65, 66, 67]
This method can be used to locate the projection of the nidus with a precision of 2 mm, facilitating excision of lesions with minimal damage to normal bone. Furthermore, it can reveal the progress of the excision and the absence of residual pathologic tissue at the end of the operation.[64, 65, 66, 68, 69]
Disadvantages of this method include the narrowness and depth of the operative field, which causes difficulty in positioning the probe perpendicular to the bone surface. In addition, narrow probes can cause false-negative readings and, hence, imprecise localization of the lesion. Also, at the end of the operation, confirmation of complete removal of the lesion is difficult because of the altered anatomy of the site. Finally, the surgeon might be unsure if the lesion is completely excised because the probe detects high radioactivity in perilesional sclerosis.[67]
CT-guided percutaneous excision
Preoperative insertion of a needle under the guidance of computed tomography (CT) is important for localizing the nidus of an osteoid osteoma during surgery. Proper insertion of the needle reduces the amount of bone removed during surgery and reduces the risk of postoperative fracture.[70] Another advantage of this procedure is immediate verification of complete removal of the nidus with histologic confirmation of the diagnosis.[38]
The technique is done with the patient under local anesthesia. It entails identification of the lesion on the CT scan. Then, with CT guidance, a Kirschner wire (K-wire) is inserted and drilled through the cortex into the nidus. A small incision is created, a biopsy punch is inserted over the K-wire, and the specimen is completely removed. Postoperative CT is performed to confirm complete evacuation of the nidus. Finally, pathologic examination is done to confirm the diagnosis.
CT-guided percutaneous resection has a success rate of 83-100%. Campanacci et al noted a primary cure rate of 83% after a percutaneous procedure, with an additional 9% cured after a second procedure. However, they reported a failure rate of 2%, with no change in pain, after percutaneous treatment.[58]
Donohue et al observed that preoperative pain resolved within 1-2 weeks after percutaneous treatment, with no clinical or radiographic evidence of recurrence at a mean follow-up of 17 months (range, 9-43 months).[71]
Engel et al studied 15 patients who underwent CT-guided percutaneous drilling. The researchers concluded that the procedure is reliable and safe, with a lower cost. However, the negative points included weakening of the bone and the need to have an orthopedic surgeon, a radiologist, and an anesthetist present simultaneously in the tomography room.[72]
Raux et al reported on 42 cases of osteoid osteoma located in the neck of the femur or the lesser trochanter that were treated by means of CT-guided percutaneous bone resection and drilling (PBRD); patients were followed for a minimum of 1 year (range, 12-56 months).[73] In 35 cases, PBRD resulted in cure, with complete and permanent pain relief.
Complications associated with this procedure include postoperative fracture, limitation of activity and impaired weight bearing for as long as 3 months after surgery, skin burns due to high rotation speed of the instrument, muscle hematoma, irritation of adjacent nerves with transient paresis, and osteomyelitis. Sans et al noted a complication rate of 24% in this procedure.[39]
Percutaneous laser photocoagulation
Bown et al first described percutaneous laser photocoagulation in 1983.[74] The technique entails CT-guided localization of the nidus. A bare optical fiber or fibers are inserted directly into the target tissue, followed by treatment with low levels of power or laser energy (2-4 W) for several minutes. This causes a relatively predictable area of coagulative necrosis secondary to thermal destruction of the nidus.[75, 76]
Witt et al quantified the amount of coagulation in relation to the amount of power applied over the lesion.[77] With 2 W of constant power, the mean axial diameter of coagulation was 3.4 mm with 200 J and 9.2 mm with 1000 J; the mean longitudinal diameter of coagulation was 4 mm with 200 J and 11.1 mm with 1400 J. Maximal effect was reached with delivery of 1000-1200 J; application of more energy yielded no alteration in the area of coagulation. Complete relief of pain was noted within 24-48 hours; at 72 hours, the patients could stop taking analgesics.[77]
Lindner et al described a primary cure rate of 93% and a 96% cure rate after the second ablation.[78]
Percutaneous radiofrequency coagulation
Percutaneous radiofrequency coagulation is performed by using an electrode placed in the lesion, coupled with a radiofrequency generator that produces local tissue destruction by converting radiofrequency into heat.
The technique involves CT-guided insertion of a trocar, followed by application of an electrode. Electrode placement is then confirmed with CT, and after confirmation, the lesion is heated to 90°C for 4 minutes. Lesions that are larger than average are treated with two passes of radiofrequency.[79]
Tillotson et al showed reproducible zones of necrosis of 0.9-1.3 cm in diameter when lesions were heated to 80°C. Neither varying the duration of heating from 30 seconds to 4 minutes nor increasing the size of the tip altered the area of necrosis. Furthermore, the extent of the lesion and, therefore, tissue necrosis did not increase over time (3 wk after the procedure).[80]
Lundskog et al showed that osteocyte necrosis occurs at 50°C sustained for 30 seconds. Blood flow limited heat transmission in bone; therefore, lethal temperature to the tissues could not be sustained over great distances.[81] Microscopic examination showed hemorrhage with a radius of approximately 5 mm extending from the probe tract. Adjacent bone was intact, and viable cells were seen.
Over the next 6 weeks, marrow fat necrosis and reactive fibrosis replaced this hemorrhage.[81] After 6 weeks, a thin, circular rim of intramedullary reactive bone surrounded this area of fat necrosis.
Complete or nearly complete relief of pain often occurs within 3 days. Patients are sent home on the day of surgery, and they have no limitations in weightbearing, though aggressive athletics are restricted for 2-3 months. Patients may then return to normal activities immediately or within 24-48 hours after surgery. Pain resolves immediately, and limping resolves within 24 hours. Furthermore, this procedure requires only a small osseous access to allow insertion of the electrode; therefore, no substantial structural weakening of the bone occurs.
Primary cure rates are 83-94%. Cure with a second ablation procedure is approximately 100%. Rosenthal et al noted no recurrences in their patients at more than 1 year after the procedure.[60, 61]
Vanderschueren et al reported factors that decrease or increase risk of treatment failures. Advanced age (mean, 24 years) and increased number of needle positions during thermocoagulation decrease the risk of treatment failure. However, young age (mean, 20 years) and a lesion of 10 mm or larger increases the risk of treatment failure.[82]
Rehnitz et al used a questionnaire to follow-up with 72 patients who underwent CT-guided radiofrequency ablation (RFA). They concluded that the long-term outcome was excellent and that findings from CT and magnetic resonance imaging (MRI) do not always correlate with the clinical outcome.[83]
Percutaneous radiofrequency coagulation is currently the preferred treatment for osteoid osteoma because it does not require hospitalization, is not associated with complications, and is associated with rapid convalescence.
The main disadvantages of this procedure are recurrence or persistence of the osteoid osteoma and the lack of histologic verification. Recurrent lesions can be managed with repeat percutaneous RFA, but lesions should be confirmed histologically by means of needle biopsy before ablation.[84, 85] Lesions that are resistant to percutaneous RFA can easily be treated with open surgery. Another complication is local skin burns.
Computer-assisted surgery
Computer-assisted surgery is a collection of techniques in which imaging and use of three-dimensional (3D) tracking devices are combined to improve surgical performance. This surgery is based on initial diagnostic CT scans that are processed into 3D images. The volume or surface is then extracted to produce a computational model of the anatomy.[71, 79, 86]
The technique entails intraoperatively registering the patient to the preoperative image by determining a rigid-body transformation. Optical trackers mounted on modified surgical instruments are attached to the patient to allow for intraoperative tracking with an optical system. Given the transformation between patient and image, the computer displays the 3D location and orientation of an instrument by superimposing a graphical representation of the instrument on the preoperative computer-generated image.
The advantage of this technique is that it can provide precise and accurate localization of lesions in bone. It is useful for small lesions located deep in the cortical bone, where there may be subtle or no surface changes to guide the surgeon. The procedure is conducted without fluoroscopy; fluoroscopy is used only at completion of the operation to document bone-tunnel placement. The percutaneous technique can be used in unison with this procedure.
Magnetic resonance–guided focused ultrasound
Magnetic resonance–guided focused ultrasound (MRgFUS) is a novel imaging-guided surgical technique that allows the performance of noninvasive and radiation-free ablation. In a prospective multicenter study by Geiger et al, 29 patients with a nonspinal osteoid osteoma were treated with MRgFUS.[87] At 12-month follow-up, technical success was achieved in all cases: complete clinical success in twenty-six (90%) and partial success in three (10%). No complications were observed.
Incomplete resection is a real complication of minimally invasive procedures. Roger et al observed incomplete resection in 35.7% of patients, as assessed by immediate follow-up scintigraphy following CT-guided percutaneous excision. These patients needed an immediate second resection to extirpate the lesion and relieve pain.[88]
Recurrence is frequently linked to incomplete resection of the tumor. Rosenthal et al[60, 61] and Barei et al[9] noted a 12% recurrence rate in patients who underwent percutaneous RFA. This rate was not significantly different from that associated with surgical excision of the nidus. Atar et al stated that this was also the case with CT-guided percutaneous excision.[23]
Rosenthal et al noted that in 23% of patients, symptoms persisted following percutaneous RFA. This was significantly lower than the 30% rate associated with operative excision.[60, 61]
Copyright © www.orthopaedics.win Bone Health All Rights Reserved