

The median nerve, colloquially known as the "eye of the hand," is one of the three major nerves of the forearm and hand. It courses from the brachial plexus in the axilla to innervate the intrinsic muscles of the hand. Median nerve entrapment syndrome is a mononeuropathy that affects movement of or sensation in the hand. It is caused by compression of the median nerve in the elbow or distally in the forearm or wrist, with symptoms in the median nerve distribution. Forms of median nerve entrapment include the following:
Cases of CTS have been reported since the late 19th century. The first carpal tunnel release was described by Learmonth in 1933. For some years to follow, only a handful of operative reports of transverse carpal ligament release were described, presumably because the diagnosis was attributed to other proximal neuropathies. The surgical technique for CTS has remained constant, with more than 95% of cases done through a small longitudinally oriented incision distal to the volar wrist crease. Although an endoscopic approach has been employed for carpal tunnel release, the open procedure remains the more popular operation.
NextThe median nerve has roots in C5, C6, C7, C8, and T1. It is formed in the axilla by the lateral and medial cords of the brachial plexus, which arise on opposite sides of the axillary artery and fuse to form the median nerve anterior to the artery (see the image below).
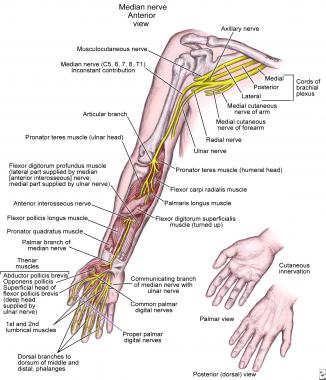 Anatomy of median nerve along its course in upper extremity.
Anatomy of median nerve along its course in upper extremity.
As the nerve courses to the elbow, it lies close to the brachial artery, crossing it anteriorly to medially. After entering the cubital fossa lateral to the brachialis tendon, the median nerve passes between the two heads of the pronator teres, a possible site of compression. As the nerve enters the forearm, it branches to the pronator teres, the flexor carpi radialis, the palmaris longus, and the flexor digitorum superficialis (FDS). The median nerve also gives off a significant branch within the pronator teres, the AIN, which supplies the flexor pollicis longus, the pronator quadratus, and the lateral half of the flexor digitorum profundus (FDP).
The median nerve continues its course in the distal forearm, under the FDS and on the FDP. The palmar cutaneous branch emerges as the median nerve becomes superficial, just above the wrist. This branch supplies the thenar eminence and central palm. After branching, the median nerve continues into the hand via the carpal tunnel. The carpal bones and the pronator quadratus compose the inferior and side borders of the carpal tunnel, and the flexor retinaculum forms the roof of the canal.
In the carpal tunnel, the median nerve runs anteriorly and laterally to the tendons of the FDS. In the hand, a muscular branch forms to supply the muscles of the thenar eminence, and the palmar digital branch forms to supply the palmar surface of the thumb, index, and middle finger and the lateral half of the ring finger, including the nail beds on the dorsal surface. The palmar nerves also give off branches to supply the two lateral lumbrical muscles.
Compression of the median nerve can occur at various sites along its course,[6, 7, 8] causing specific and variable signs and symptoms.
CTS is the most common of the median nerve entrapments. The carpal tunnel is a narrow fibro-osseous tunnel through which the median nerve passes, along with nine tendons. An increase in the volume of the tunnel contents or a decrease in the size of the tunnel can compress the median nerve.
The ligament of Struthers (see the image below) is usually the most proximal site of compression. An anomalous bony spur, the supracondylar process, is located at the distal humerus, approximately 3-5 cm proximal to the medial epicondyle and 2-20 mm long.[9] The ligament of Struthers connects the supracondylar process to the medial epicondyle, encasing the median nerve and brachial artery. It is seen in approximately 13% of the general population and rarely causes median nerve entrapment.[10] In some cases, no bony spur can be identified; only the ligament persists.[11, 12]
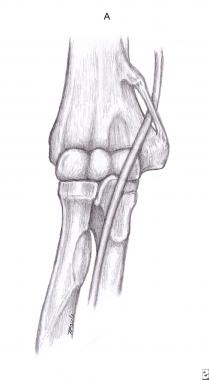 Ligament of Struthers.
Ligament of Struthers.
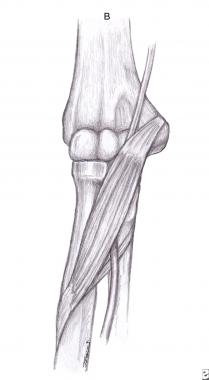
The lacertus fibrosus (bicipital aponeurosis; see the image below) is the least common cause of pronator syndrome.[13, 14] The bicipital aponeurosis is the medial extension of the biceps tendon and covers the median nerve in the cubital fossa. The compression may be secondary to hypertrophy or enlargement of the aponeurosis.
 Lacertus fibrosus (bicipital aponeurosis).
Lacertus fibrosus (bicipital aponeurosis).
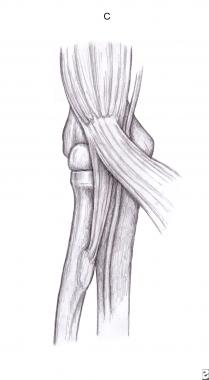
Fibrous bands between the deep and superficial heads of the pronator teres (see the image below) frequently cause compression of both the median nerve and the AIN. An anatomic study proposed the determinant variations that can lead to pronator syndrome: a short and tendinous ulnar head, an ulnar head joined to the arch of the FDS muscle, an ulnar head with triple origin slips, and a humeral head perforated by the median nerve.[15]
 Pronator teres.
Pronator teres.
The FDS varies in origin and size, and the median nerve can be crossed and compressed by one or two aponeurotic arches (see the image below).[16, 17]
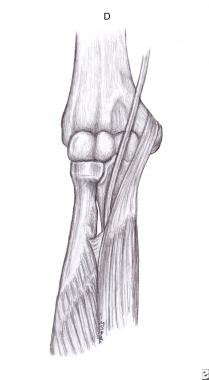 Fibrous arch of flexor digitorum superficialis.
Fibrous arch of flexor digitorum superficialis.
The anterior interosseous branch of the median nerve is subject to compromise near its origin. This syndrome usually occurs spontaneously, but can be caused by fracture, fibrous bands, aberrant or thrombosed vessels, and tumors. An existing anatomical abnormality, whether congenital or traumatic, can increase the predisposition to the development of anterior interosseous nerve syndrome (AINS), especially if the area is challenged with concurrent localized edema. Known sites of compression include the following:
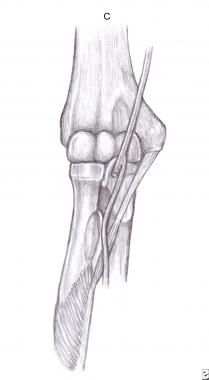 Gantzer's muscle.
Gantzer's muscle.
Increased pressure in the carpal tunnel blocks venous blood flow and axonal transport; higher pressures block intraneural blood flow and impede conduction. These pressures can be measured directly by catheters placed in the carpal tunnel. Normal pressures are in the range of 2-10 mm Hg. This pressure is affected by finger, wrist, and forearm position.[20] Wrist extension causes the greatest increase in carpal pressure. High pressures result in complete block of nerve conduction.
Median nerve compression is also associated with decreased space in the carpal canal, which can be caused by increased edema and inflammation of tenosynovium seen in systemic conditions such as diabetes,[21] arthritis, thyroid dysfunction, and renal failure. Patients with diabetes have a higher tendency to develop CTS as a consequence of their lower threshold for nerve damage.
Most of the information on chronic compressive neuropathies is based on animal models because of the paucity of biopsy studies on human nerves.
In a rare histologic study of nerves performed in a patient with CTS who died of a brain tumor, the authors found extensive demyelination and remyelination in the entrapped nerve.[22] Large myelinated axons were significantly decreased. Proximal nerve swelling associated with perineurial and endoneurial fibrosis was demonstrated. This correlates with the observation during surgery of thinned nerves in the zone of entrapment with proximal swelling. Animal models in which chronic neural compression is stimulated by banding of nerve with Silastic tubes have yielded similar results.[23]
Initial changes included perineurial thickening and peripheral segmental demyelination. Later changes were progressive epineurial and perineurial thickening and global demyelination. Progressive slowing of conduction velocity after an initial increase also occurred.
Both ischemic and mechanical factors have been postulated in the development of compression neuropathy. Acute and chronic compression of peripheral nerves can induce changes in intraneural microcirculation and nerve-fiber structure, increase vascular permeability with subsequent edema formation, and impair anterograde and retrograde axonal transport, which all contribute to the clinical symptoms and deterioration of nerve function.[23, 24]
According to Mackinnon,[25] "Experimental studies suggest a dose-response curve such that the greater the duration and amount of pressure, the more significant is neural dysfunction." The main electrophysiologic finding in patients with symptomatic CTS is prolonged latency, indicating demyelination. However, the extent of demyelination correlates very poorly with clinical symptoms. This poor correlation may be explained by the vascular component of the pathophysiology. Chronic mechanical trauma causes fibrosis of the perineurium and epineurium. This induces vascular proliferation, vascular hypertrophy, and vascular obstruction with wall thickening and reduction in elastin content.[26]
Although endoneurial capillaries normally constitute a blood-neural barrier (BNB) that helps to optimize the endoneurial environment, damage to the vessels may induce a miniature closed compartment syndrome by increasing the permeability, thereby contributing to increased endoneurial fluid pressure and development of an intrafascicular edema.[27] In addition, neural gliding is prevented because of neural tethering, which decreases the excursion of the nerve fibers and results in traction. This is the basis of the tethered median nerve stress test (TMNST), which is sometimes used to diagnose chronic low-grade CTS.[28]
Upton and McComas introduced the double-crush concept of chronic nerve compression. The hypothesis suggests that compression at one site along the course of the nerve makes it more susceptible to compression at another site by compromising the axoplasmic flow in the nerve.[29] Similarly, the reverse double-compression theory[30] states that compression of the nerve at the distal site would decrease transport of neurotrophic substances to the proximal site, thus reducing its overall production. Potential secondary sites of compression include the cervical spine, the thoracic outlet, and the cubital fossa. Animal studies have further corroborated this hypothesis.[31, 32]
Investigators are also looking into the biochemical aspects of chronic compressive neuropathies. One study found increased expression of prostaglandin E2 and vascular endothelial growth factor (VEGF) in synovial biopsy tissues from patients with a symptomatic CTS duration history of 5-7 months and more than 12 months (but not 8-12 months).[33] The same authors found increased expression of matrix metalloprotein 2 in small arterioles during the early painful phase of CTS.[34]
Another study involving 30 patients with variable intensity and symptom duration found a marked increase in fibroblast density, collagen fiber size and vascular proliferation, type III collagen, and increased expression of transforming growth factor (TGF)-β in fibroblasts, suggesting a response to injury to the subsynovial connective tissue.[35]
Risk factors have been well studied predominantly for CTS only, with the other types of median nerve entrapment being generally classified as idiopathic. The incidence of some of these risk factors differs in various studies, but most populations with CTS have somewhat similar rates of associated risk factors.[36]
According to de Krom et al,[37] associated factors include activities with a flexed wrist or with an extended wrist (exposure-related increased risk), hysterectomy without oophorectomy, height, weight, Quetelet index, slimming courses, and, in men, varicosis. However, they did not find any association between CTS and use of oral contraceptives, age at menopause, diabetes, thyroid dysfunction, rheumatism, typing, or pinch grasp.
In a retrospective study of 1016 residents of Rochester, Minnesota, who were diagnosed with CTS from 1961 through 1980, 43.2% had no associated conditions.[38] The most frequent associated conditions included Colles fracture, rheumatoid arthritis, hormonal agents or oophorectomy (or both), diabetes mellitus, and, among men, occupations that involved excessive use of the hands. Rheumatoid arthritis, diabetes mellitus, and pregnancy were significantly more frequent among the study patients with CTS than in the general population of Rochester.
These findings were supported by a retrospective case control study of 514 patients who underwent carpal tunnel release and 100 matched cases at the University of Washington.[39] The investigators of this study concluded that CTS is multifactorial, with such factors as obesity, hypothyroidism, and diabetes (but not smoking) more prevalent in the study group of patients with CTS than in the matched controls.
In a population-based case control study in Britain,[40] the authors found risk factors associated with CTS included previous wrist fracture; rheumatoid arthritis; osteoarthritis of the wrist and carpus; obesity; diabetes; and the use of insulin, sulfonylureas, metformin, or thyroxine. No association was found between CTS and smoking, hormone replacement therapy, or combined use of oral contraceptive pill and oral corticosteroids. The results were similar when cases were restricted to those who had undergone carpal tunnel decompression. One study found increased CTS prevalence in patients with untreated hyperthyroidism, which remitted after successful treatment of the medical condition.[41]
Overall, the etiology of CTS is generally accepted as idiopathic, in that the findings described above are considered to be based on unproven theories. More information about the etiology of idiopathic carpal tunnel syndrome is needed. This might reveal new ways of preventing or treating this condition.
Because median nerve entrapment encompasses various distinct syndromes, its exact prevalence is unknown.
Most of the epidemiologic studies involving median nerve compressive neuropathy have centered on CTS, which is the most common peripheral nerve entrapment syndrome, with a prevalence of 5.8% in women and 0.6% in men, according to a European study.[37] The estimated incidence of CTS in the United States is 3.4%.[42]
Another study found that one of five symptomatic subjects would be expected to have CTS on the basis of clinical examination and electrophysiologic testing. The use of highly repetitive wrist movements, vibrating tools, awkward wrist positions, or great force seems to predispose to CTS, though the exact cause of CTS is a matter of controversy.[36] In a study on incidence of CTS in automobile workers, the annual incidence of CTS was found to be 1-10%.[43] Prevalence in some industries, such as fish processing, has been reported to be as high as 73%.[44]
Other conditions that are associated with a high risk for CTS include diabetes and pregnancy.[21, 45] People with diabetes have been known to have prevalence rates of 14% and 30% without and with diabetic neuropathy, respectively.[46] Prevalence of CTS during pregnancy has been reported to be around 2%.[47]
The exact incidence of proximal median nerve entrapment in the population is less well understood than that of CTS. On average, proximal median nerve compression syndrome accounts for less than 1% of upper-extremity compressive syndromes.[48] However, some studies report a very high incidence of pronator syndrome in already symptomatic workers; for example, female machine milkers have rates as high as 75%.[49] The incidence of pronator syndrome is four times higher in women than in men.
In 1966, Cseuz et al[50] found that duration of symptoms, presence of associated diseases, and extent of abnormality found on median nerve conduction studies were not good predictors of the outcome of carpal tunnel surgery.
Patients who obtain transient symptom relief from steroid injection of the carpal tunnel are more likely to benefit from carpal tunnel surgery than are patients who do not obtain relief from steroid injection.[51, 52]
Relief of intermittent paresthesias and pain, some improvement in the sensibility of the fingers, and improvement of nocturnal symptoms are often immediate or evident as soon as incisional pain resolves. After the initial and usually dramatic improvement in sensibility, the patient experiences some slight decrease in improvement (~1 week postoperatively). Improvement will again be realized after this transient partial relapse.
The changes in the conduction velocity seem to parallel the patient's clinical response. It has been demonstrated intraoperatively that an immediate improvement in conduction velocity can be anticipated in most cases of carpal tunnel release.[53] Patients who have no associated symptoms of thoracic outlet syndrome and/or physically laborious work activities are most likely to benefit from carpal tunnel release.[54]
After carpal tunnel release, swelling at the base of the palm superficial to the carpal tunnel can be expected to persist for 12-16 weeks. During this period, and probably concurrent with the swelling, the patient may experience aching pain in the thenar or hypothenar eminences, also known as "pillar pain."
In most cases, strength slowly returns after carpal tunnel release. By 6 weeks after surgical release of the deep transverse carpal ligament, patients usually have only about 50% of their preoperative grip strength. As long as patients are able to exercise and work effectively on increasing grip strength, which should begin in postoperative week 2 or 3, 75% of grip strength is usually regained by postoperative week 8-10. Maximum grip strength and, perhaps more important, endurance of grip strength often do not return until 6 months or even longer after surgical release of the carpal tunnel. As many as 20% of patients never regain full strength after surgical release of the transverse carpal ligament.
The side effects of carpal tunnel release, their causes, and rates of resolution are debated in the literature. That the volume of the tunnel is increased is generally agreed. That the grip strength decreases is also agreed, but its speed of recovery varies widely, from 3 to 9 months. Whether the arch widens is disputed, as is the importance of that widening. Sensation generally improves rapidly after simple release.
Clinical Presentation
Copyright © www.orthopaedics.win Bone Health All Rights Reserved